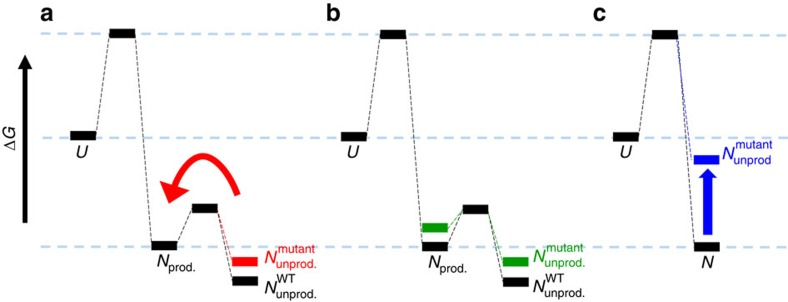
Figure 7. Putative effects of mutations on the energy landscape of IGPS.
Free energy diagrams showing three possible scenarios for the effect of a single mutation on the folding free energy surface (ΔG°, Gibbs free energy of folding; U, unfolded state; N, native state ensemble). An ensemble of rapidly interconverting productive (Nprod.) and nonproductive (Nunprod.) conformations resides in the native basin. At mesophilic temperatures, thermophilic IGPS access the productive conformation to a lesser extent than at their native thermophilic temperatures. (a) Beneficial mutations may destabilize the unproductive native state without a concomitant destabilization of the higher energy, productive conformation, resulting in a shift in the population from the unproductive to the productive conformation. Increased activity improves fitness. (b) WT-like mutations may destabilize both the unproductive and productive states, resulting in no net change in the population. No change in fitness is observed. (c) Deleterious mutations may greatly destabilize the native state, resulting in a population shift to inactive, partially or fully unfolded, states that are susceptible to proteolysis. Poor fitness is associated with loss of enzymatic capabilities.