Abstract
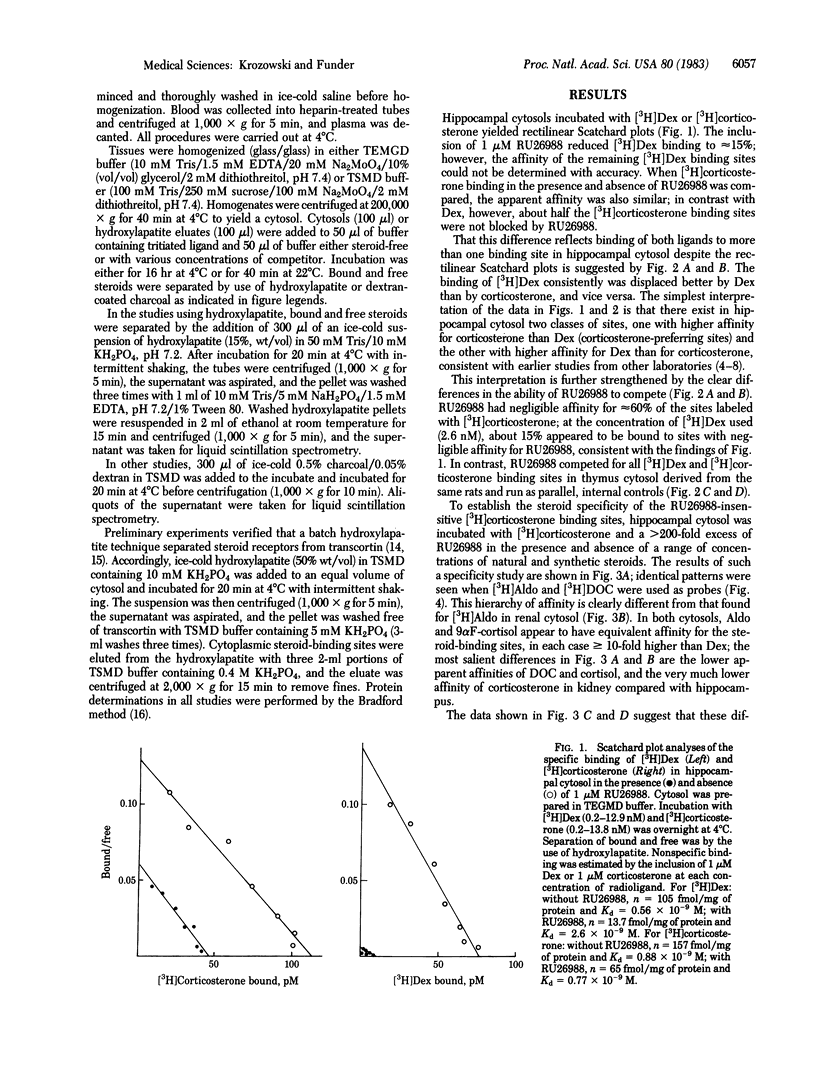
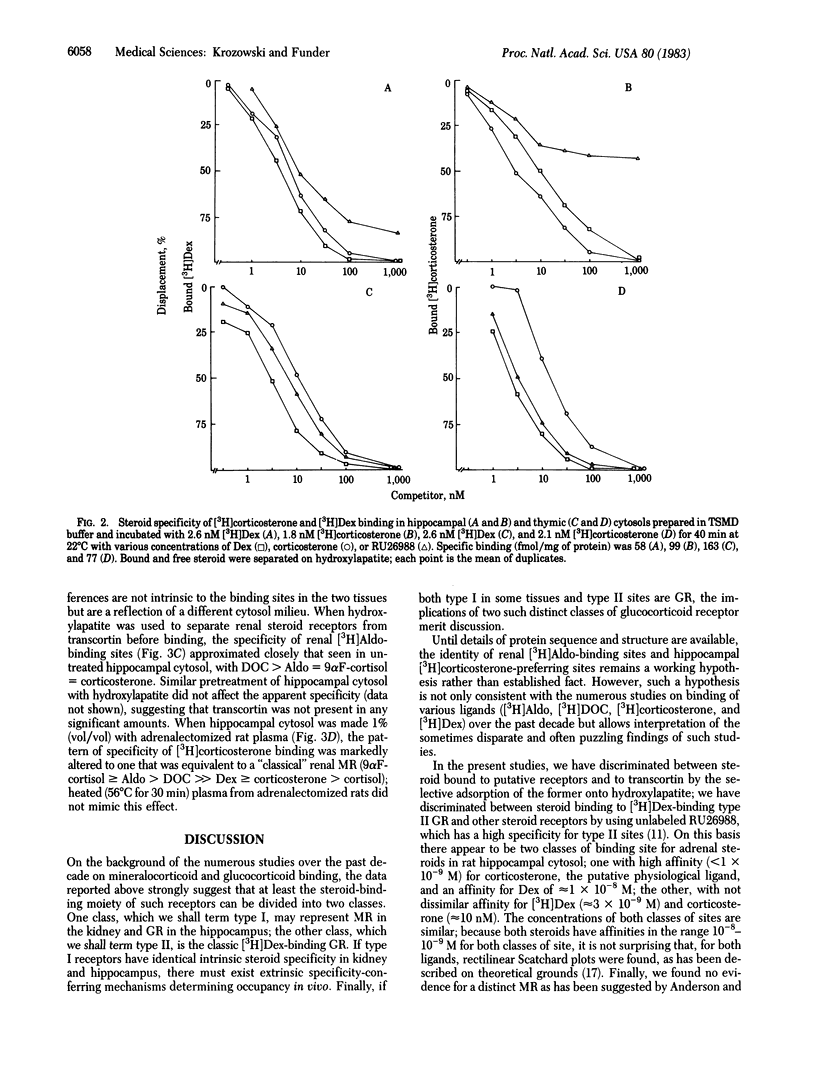
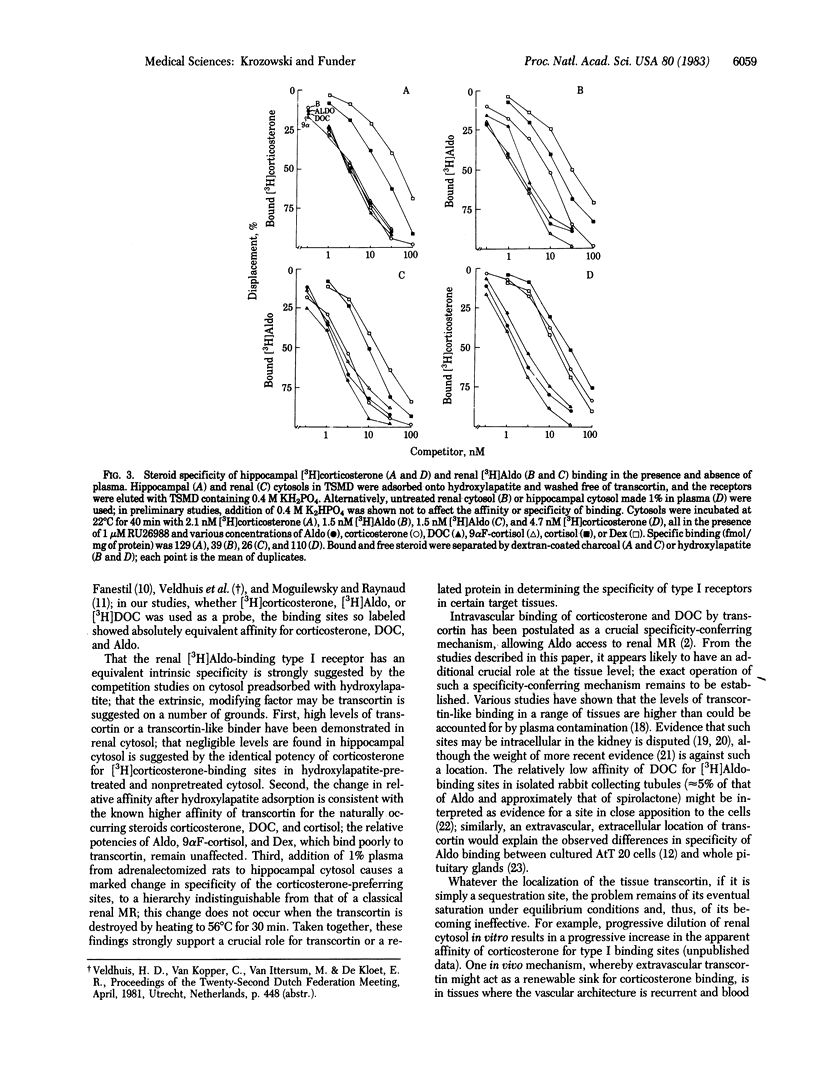
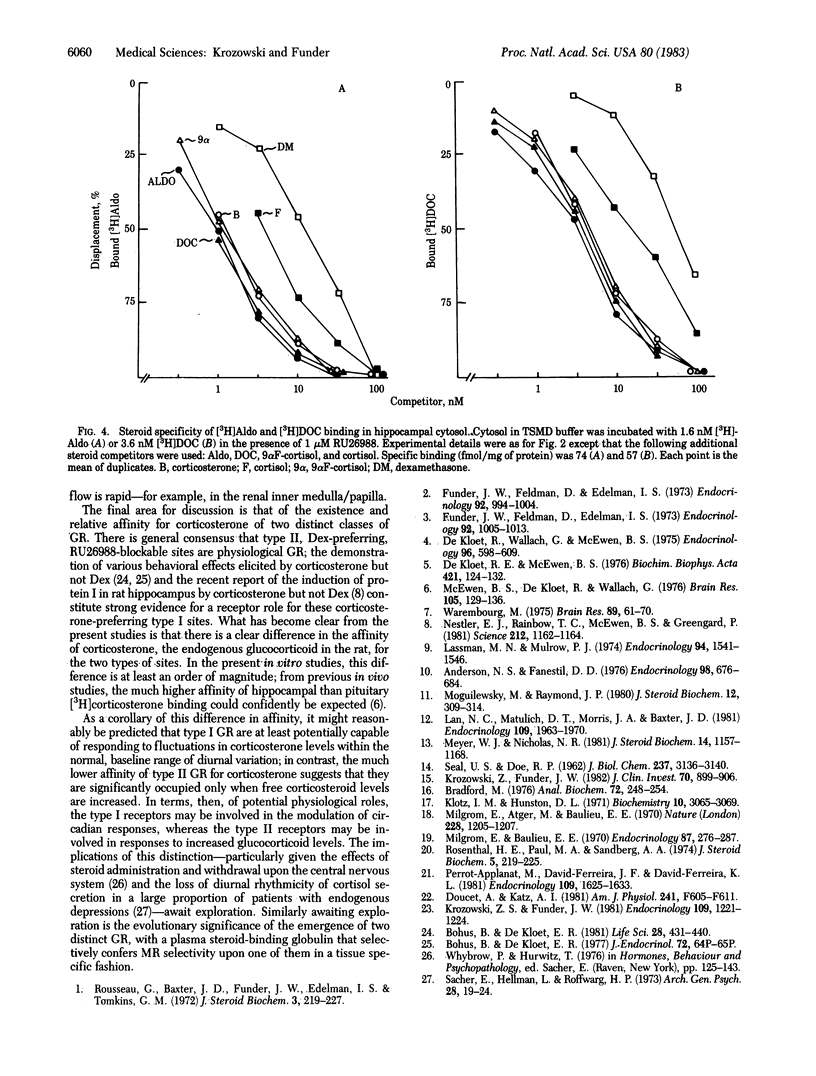
There is current evidence for two classes of hippocampal glucocorticoid receptors (GR)--one classical, [3H]dexamethasone [( 3H]Dex)-binding sites in glial cells, and the other [3H]corticosterone-preferring sites in neuronal cells. In the presence of 1 microM of the synthetic glucocorticoid RU26988 (11 beta, 17 beta-dihydroxy-17 alpha-propynylandrost-1,4,6,-trien-3-one) to exclude tracer from [3H]Dex sites, hippocampal cytosol from adrenalectomized/ovariectomized Sprague-Dawley rats binds [3H]Dex to sites (Kd at 4 degrees C, 0.77 X 10(-9) M; 65 fmol/mg of protein) with the following order of specificity: aldosterone (Aldo) = 9 alpha-fluorocortisol (9 alpha F-cortisol) = deoxycorticosterone (DOC) = corticosterone greater than cortisol much greater than Dex; [3H]Aldo, [3H]DOC, and [3H]corticosterone binding show identical specificity in the presence of RU26988. Addition of 1% adrenalectomized/ovariectomized rat plasma (but not plasma heated at 56 degrees C for 30 min) alters the specificity to: 9 alpha F-cortisol greater than or equal to Aldo greater than or equal to DOC much greater than Dex greater than or equal to corticosterone greater than or equal to cortisol, consistent with sequestration of DOC, corticosterone, and cortisol by transcortin and similar to classical mineralocorticoid receptor (MR) binding of [3H]Aldo in renal cytosol (9 alpha F-cortisol greater than or equal to Aldo greater than or equal to DOC much greater than corticosterone greater than or equal to cortisol greater than or equal to Dex). Separation of other renal binders from transcortin by hydroxylapatite adsorption established the intrinsic specificity of [3H]Aldo binding to MR as: DOC greater than or equal to Aldo greater than or equal to 9 alpha F-cortisol greater than or equal to corticosterone greater than cortisol much greater than Dex, parallel to that of the [3H]corticosterone-binding sites in hippocampus. These studies suggest (i) that hippocampal [3H]corticosterone-binding sites and renal MR may have identical intrinsic specificity for steroids, with apparent specificity differences the result of tissue-specific sequestration of naturally occurring steroids other than Aldo and (ii) that an identical steroid-binding species may thus be occupied under physiological conditions by a mineralocorticoid in one tissue (kidney) and a glucocorticoid in another (hippocampus).
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. S., 3rd, Fanestil D. D. Corticoid receptors in rat brain: evidence for an aldosterone receptor. Endocrinology. 1976 Mar;98(3):676–684. doi: 10.1210/endo-98-3-676. [DOI] [PubMed] [Google Scholar]
- Bohus B., de Kloet E. R. Adrenal steroids and extinction behavior: antagonism by progesterone, deoxycorticosterone and dexamethasone of a specific effect of corticosterone. Life Sci. 1981 Jan 26;28(4):433–440. doi: 10.1016/0024-3205(81)90090-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- De Kloet E. R., McEwen B. S. Differences between cytosol receptor complexes with corticosterone and dexamethasone in hippocampal tissue from rat brain. Biochim Biophys Acta. 1976 Jan 14;421(1):124–132. doi: 10.1016/0304-4165(76)90176-8. [DOI] [PubMed] [Google Scholar]
- De Kloet R., Wallach G., McEwen B. S. Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology. 1975 Mar;96(3):598–609. doi: 10.1210/endo-96-3-598. [DOI] [PubMed] [Google Scholar]
- Doucet A., Katz A. I. Mineralcorticoid receptors along the nephron: [3H]aldosterone binding in rabbit tubules. Am J Physiol. 1981 Dec;241(6):F605–F611. doi: 10.1152/ajprenal.1981.241.6.F605. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Edelman I. S. Glucocorticoid receptors in rat kidney: the binding of tritiated-dexamethasone. Endocrinology. 1973 Apr;92(4):1005–1013. doi: 10.1210/endo-92-4-1005. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Edelman I. S. The roles of plasma binding and receptor specificity in the mineralocorticoid action of aldosterone. Endocrinology. 1973 Apr;92(4):994–1004. doi: 10.1210/endo-92-4-994. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Hunston D. L. Properties of graphical representations of multiple classes of binding sites. Biochemistry. 1971 Aug 3;10(16):3065–3069. doi: 10.1021/bi00792a013. [DOI] [PubMed] [Google Scholar]
- Krozowski Z. S., Funder J. W. Rat anterior pituitary. Distinction of an approximately 8S, corticosterone-preferring species from dexamethasone-binding glucocorticoid receptors. J Clin Invest. 1982 Oct;70(4):899–905. doi: 10.1172/JCI110686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krozowski Z., Funder J. W. Mineralocorticoid receptors in rat anterior pituitary: toward a redefinition of "mineralocorticoid hormone". Endocrinology. 1981 Oct;109(4):1221–1224. doi: 10.1210/endo-109-4-1221. [DOI] [PubMed] [Google Scholar]
- Lan N. C., Matulich D. T., Morris J. A., Baxter J. D. Mineralocorticoid receptor-like aldosterone-binding protein in cell culture. Endocrinology. 1981 Dec;109(6):1963–1970. doi: 10.1210/endo-109-6-1963. [DOI] [PubMed] [Google Scholar]
- Lassman M. N., Mulrow P. J. Deficiency of deoxycorticosterone-binding protein in the hypothalamus of rats resistant to deoxycorticosterone-induced hypertension. Endocrinology. 1974 Jun;94(6):1541–1546. doi: 10.1210/endo-94-6-1541. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., de Kloet R., Wallach G. Interactions in vivo and in vitro of corticoids and progesterone with cell nuclei and soluble macromolecules from rat brain regions and pituitary. Brain Res. 1976 Mar 19;105(1):129–136. doi: 10.1016/0006-8993(76)90928-8. [DOI] [PubMed] [Google Scholar]
- Meyer W. J., 3rd, Nichols N. R. Mineralocorticoid binding in cultured smooth muscle cells and fibroblasts from rat aorta. J Steroid Biochem. 1981 Nov;14(11):1157–1168. doi: 10.1016/0022-4731(81)90046-7. [DOI] [PubMed] [Google Scholar]
- Milgrom E., Atger M., Baulieu E. E. Progesterone binding plasma protein (PBP). Nature. 1970 Dec 19;228(5277):1205–1206. doi: 10.1038/2281205a0. [DOI] [PubMed] [Google Scholar]
- Milgrom E., Baulieu E. E. Progesterone in uterus and plasma. I. Binding in rat uterus 105,000 g supernatant. Endocrinology. 1970 Aug;87(2):276–286. doi: 10.1210/endo-87-2-276. [DOI] [PubMed] [Google Scholar]
- Moguilewsky M., Raynaud J. P. Evidence for a specific mineralocorticoid receptor in rat pituitary and brain. J Steroid Biochem. 1980 Jan;12:309–314. doi: 10.1016/0022-4731(80)90285-x. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Rainbow T. C., McEwen B. S., Greengard P. Corticosterone increases the amount of protein 1, a neuron-specific phosphoprotein, in rat hippocampus. Science. 1981 Jun 5;212(4499):1162–1164. doi: 10.1126/science.6785886. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M., David-Ferreira J. F., David-Ferreira K. L. Immunocytochemical localization of corticosteroid-binding globulin (CBG) in guinea pig hepatocytes. Endocrinology. 1981 Nov;109(5):1625–1633. doi: 10.1210/endo-109-5-1625. [DOI] [PubMed] [Google Scholar]
- Rosenthal H. E., Paul M. A., Sandberg A. A. Transcortin: a corticosteroid-binding protein of plasma. XII. Immunologic studies on transcortin in guinea-pig tissues. J Steroid Biochem. 1974 May;5(3):219–225. doi: 10.1016/0022-4731(74)90135-6. [DOI] [PubMed] [Google Scholar]
- Rousseau G., Baxter J. D., Funder J. W., Edelman I. S., Tomkins G. M. Glucocorticoid and mineralocorticoid receptors for aldosterone. J Steroid Biochem. 1972 Feb;3(2):219–227. doi: 10.1016/0022-4731(72)90053-2. [DOI] [PubMed] [Google Scholar]
- SEAL U. S., DOE R. P. Corticosteroid-binding globulin. I. Isolation from plasma of diethylstilbestrol-treated men. J Biol Chem. 1962 Oct;237:3136–3140. [PubMed] [Google Scholar]
- Sachar E. J., Hellman L., Roffwarg H. P., Halpern F. S., Fukushima D. K., Gallagher T. F. Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiatry. 1973 Jan;28(1):19–24. doi: 10.1001/archpsyc.1973.01750310011002. [DOI] [PubMed] [Google Scholar]
- Warembourg M. Radioautographic study of the rat brain after injection of [1,2-3H]corticosterone. Brain Res. 1975 May 16;89(1):61–70. doi: 10.1016/0006-8993(75)90133-x. [DOI] [PubMed] [Google Scholar]


