Abstract
Aims and Objectives:
The purpose of this study was to estimate and assess any correlation between random capillary blood glucose (RCBG) and unstimulated whole salivary glucose (UWSG), as well as to estimate various salivary parameters, such as flow rate, pH, buffering capacity, and the influence of these factors on the oral health status in type 2 diabetes mellitus (DM).
Materials and Methods:
Sixty individuals suffering from type 2 DM and 40 healthy individuals in the age group of 30–60 years were included in the study. RCBG was estimated using glucometer and UWSG was estimated using photocolorimeter. Salivary parameters such as flow rate, pH, and buffering capacity were assessed using GC® Saliva kit. Oral health status was recorded using the Russell's periodontal index (RPI) and the Decayed Missing Filled Teeth (DMFT) index. The Statistical Package for the Social Sciences version 16 was used for statistical analysis.
Results:
Type 2 diabetics had higher mean values for RCBG levels and UWSG. Type 2 diabetics had low mean salivary flow rate, pH, and buffering capacity. Type 2 diabetics had higher mean values for RPI.
Conclusion:
Among the salivary factors studied, salivary glucose significantly influenced the periodontal status in Type 2 diabetics.
KEYWORDS: Buffering capacity, flow rate, pH, Type 2 Diabetes mellitus, unstimulated whole salivary glucose
INTRODUCTION
Diabetes mellitus (DM) is a clinical syndrome characterized by hyperglycemia because of absolute or relative deficiency of insulin.[1] The diagnosis of DM is based on blood glucose estimations. Blood collection is an invasive procedure, and may be traumatizing, especially in diabetic patients who require routine daily monitoring of blood glucose levels. Ongoing research in the past few decades has focussed on alternative methodologies that involve incorporating various other body fluids that could be used as a substitute for blood for diagnostic purposes. One of the most important among these is saliva. Alterations in the salivary flow and composition of saliva in diabetics have been reported in numerous previous studies, although the findings have frequently been contradictory. There still is no consensus about which parameters should be followed in saliva of type 2 DM patients to enable a salivary diagnosis of type 2 DM.[2,3,4]
MATERIALS AND METHODS
Ethical clearance for the study was obtained from the Institutional Review Board. Participants were informed about the study protocol, and only those who provided their written consent were included in the study. This cross-sectional study was conducted over a period of 8 months from June 2010 to February 2011. Based on the available literature of cross-sectional, observational studies which included sample sizes that ranged 40–180, in this study the sample size was considered to be 100, which included 60 diabetics and 40 healthy controls.[5,6,7,8,9,10,11]
INCLUSION CRITERIA
Sixty patients previously diagnosed with type 2 DM and with no other systemic illness, and 40 healthy volunteers with no apparent medical history in the age group of 30–60 years were randomly selected and included in the study.
EXCLUSION CRITERIA
Completely edentulous patients
Patients with any oral mucosal lesions
Patients on any medications other than for type 2 DM
Tobacco/betel chewing habits.
Random capillary blood glucose (RCBG) was estimated using sterile lancets and SD check Gold® Glucometer with glucose reagent strips using the finger prick method. Saliva samples were collected in the morning between 9 AM and 12 PM. Before collecting the saliva samples, patients were asked to rinse their mouth with 200 ml water. Patients were seated in an upright position during saliva sample collection. They were asked to spit into the graduated disposable collecting cup at the end of every minute for 5 minutes and the average was calculated to estimate the Unstimulated whole salivary flow rate. Salivary pH was estimated by placing the GC® saliva pH strip for 10 seconds in the saliva sample, which was then removed and matched with the color-coded table provided along with the kit. Salivary buffering capacity was estimated using the GC® Saliva buffer strips. Pipettes provided along with the kit were used to draw saliva sample from the collecting cup and 3 drops added over the 3 slots of the strips. After 2 minutes, the color change was matched with the color-coded table scores provided by the manufacturer. Periodontal status was assessed according to the Russell's periodontal index (RPI). Decayed Missing Filled Teeth (DMFT) index was recorded to assess the status of teeth.
Using a micropipette, 10 µl of saliva was drawn from the disposable collecting cup and added into a cuvette to which 1000 µl of glucose oxidase–peroxidase enzyme reagent was added; the sample was then incubated at 37°C for 10 minutes. Similarly, 10 µl of standard glucose solution was drawn into a cuvette to which 1000 µl of enzyme reagent was added, and the sample was incubated at 37°C for 10 minutes. The optical absorbance readings were recorded using the Digital photocolorimeter using the green filter with a peak of 540 nm wavelength [Figure 1].
Figure 1.
Armamentarium used to assess unstimulated whole salivary glucose
UWSG was calculated using the formula:
Salivary glucose in mg/dl = Absorbance of sample × Concentration of standard/absorbance of standard
Concentration of the standard glucose was 100 mg/dl
Statistical analysis was done using contingency coefficient analysis, independent samples t-test, multivariate analysis of variance (MANOVA), and correlations using Pearson coefficient. The Statistical Package for the Social Sciences (SPSS) for Windows (SPSS, version 16.0, Chicago, SPSS Inc.) was used for statistical analysis.
RESULTS
The mean RCBG and UWSG levels in type 2 diabetics were 180 mg/dl and 12.9 mg/dl, respectively [Table 1]. The mean RCBG and UWSG levels in healthy controls were 95.1 mg/dl and 9.46 mg/dl, respectively [Table 1]. The difference between the groups was statistically significant (P = 0.000) [Table 2].
Table 1.
Descriptive statistics
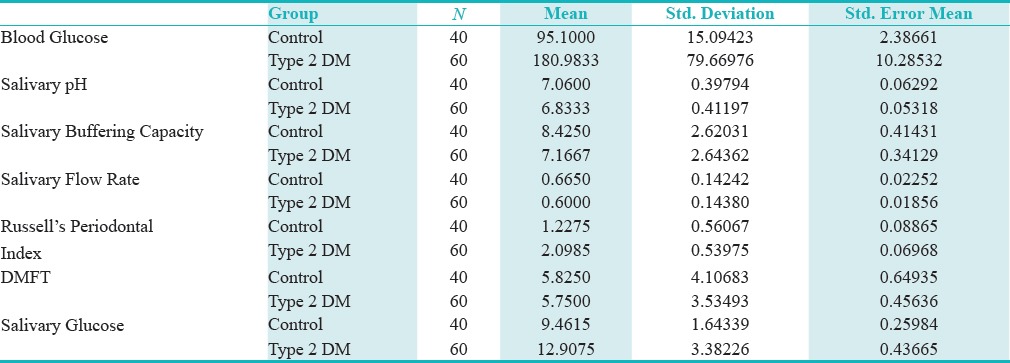
Table 2.
Independent samples test: Control and type 2 DM
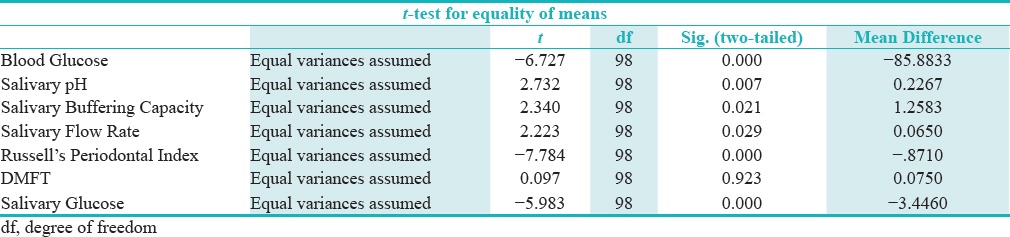
A positive correlation between RCBG and UWSG was observed in both the study and control groups [Graphs 1 and 2]. The mean unstimulated whole salivary flow rate in type 2 diabetics was 0.6 ml/min and in the healthy controls it was 0.67 ml/min [Table 1]. The difference in unstimulated whole salivary flow rate between the groups was statistically significant (P = 0.029) [Table 2]. The mean unstimulated salivary pH in type 2 diabetics was 6.8 and in the healthy controls it was 7.1 [Table 1]. The difference between the groups was significant statistically (P = 0.007).
Graph 1.
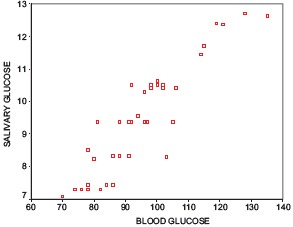
Scatter plot No.1 correlation between random capillary blood glucose and unstimulated whole salivary glucose in control group
Graph 2.
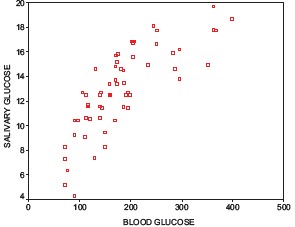
Scatter plot No 2: Correlation between random capillary blood glucose and unstimulated whole salivary glucose in experimental group
The mean salivary buffering capacity in type 2 diabetics was 7 and in the healthy controls it was 8.4 [Table 1]. There was a statistically significant difference in the salivary buffering capacity between the groups (P = 0.021) [Table 2].
No significant correlation between salivary flow rate and the salivary buffering capacity was observed in type 2 diabetics, however, a statistically significant correlation (P = 0.000) was found between salivary flow rate and salivary pH [Table 3].
Table 3.
Correlations in the study group (Type 2 diabetics and healthy volunteers) among various parameters
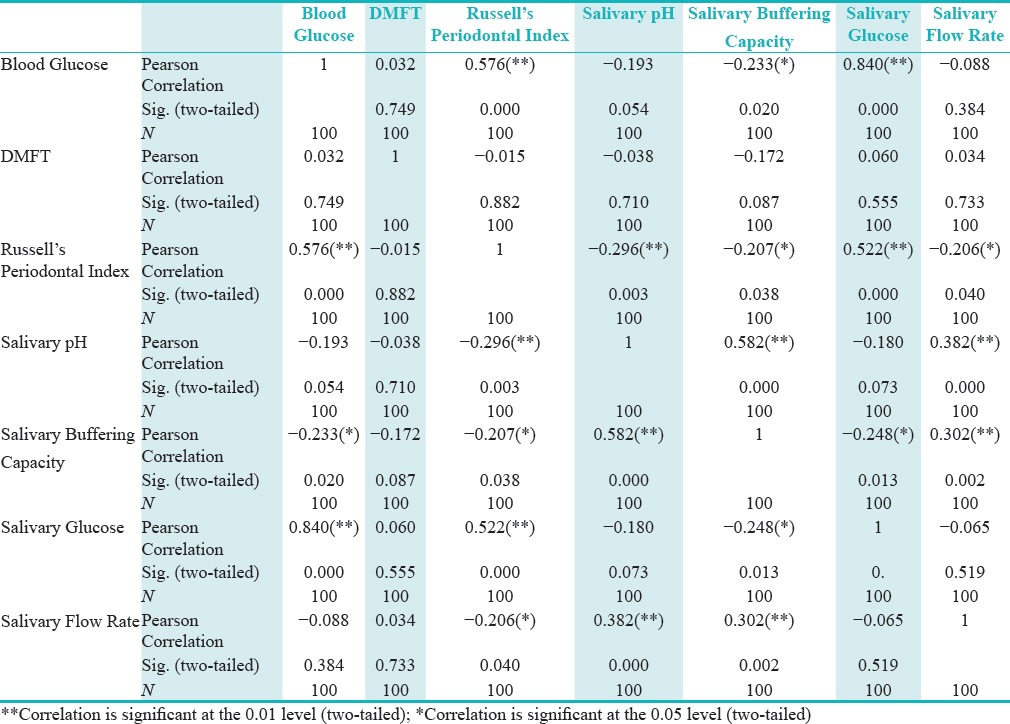
The values of salivary pH and salivary buffering capacity showed good correlation in both the type 2 diabetics and the control group, as the salivary pH decreased the salivary buffering capacity also decreased and the relationship was highly significant statistically (P = 0.000) [Table 2].
The mean values for RPI in the type 2 diabetics was found to be 2.1, and in the healthy controls it was 1.2 [Table 1]. The difference in the RPI scores between the groups was significant statistically (P = 0.000) [Table 2].
The mean DMFT scores in type 2 diabetics was 5.7 and in the healthy controls it was 5.8 [Table 1]. The difference in the DMFT scores between the groups was not significant statistically.
The RPI scores of type 2 diabetics showed positive correlation with only the salivary glucose levels among the various tested parameters. None of the tested salivary factors showed any statistically significant effect on the RPI scores in the control group.
The caries experience of both type 2 diabetics and controls in our study was similar with no statistical difference in the DMFT scores [Table 3].
DISCUSSION
Epidemiological studies in India have shown high prevalence of type 2 DM; in the year 2002, it was estimated that there were 19.4 million individuals affected by type 2 DM, which is likely to increase up to 57.2 million by the year 2025.[3] Routine blood examination for glucose assessment can be traumatizing to the patient, and hence, other alternatives have been explored, among which salivary diagnostics hold much promise. Saliva-based diagnostics are not limited to oral diseases but have been extended to the entire physiologic system, as most compounds found in the blood are also present in the saliva. Accordingly, saliva can reflect the physiologic state of the body including emotional, endocrinal, nutritional, and metabolic variations, and acts as a source for monitoring oral and systemic health.[4]
A systematic review of previously published studies reflects the fact that salivary glucose concentration increases in type 2 DM, and a positive correlation exists between blood glucose and salivary glucose; hence, it can be a useful biomarker to monitor type 2 DM.[12] In the present study, UWSG was found to reflect the RCBG levels in both the groups. Type 2 diabetics had significantly higher USWG/RCBG levels than the controls, a fact which has been documented in previous studies.[5,13,14,15,16,17,18,19] The correlation between RCBG and UWSG could plausibly be because of leakage of glucose from blood across the basement membrane of salivary glands. Microvascular alterations in the blood vessels that are commonly seen in type 2 diabetics could also contribute to increased salivary glucose levels.[20,21] Saliva samples collected in the present study represented the whole mouth fluid, and therefore, reflects glucose levels not only due to leakage across the basement membrane of major and minor salivary glands but also from the gingival crevicular fluid. Furthermore, it has been proposed by Belazi[22] that the basement membrane alterations lead to enhanced leakage of serum components including glucose into the gingival crevicular fluid rather than into saliva. However, in contrast to the present study, various other authors[13,23,24] could not establish any correlation between RCBG and UWSG.
The decrease in the unstimulated whole salivary flow rate in type 2 diabetics is in accordance with previous studies.[8,10,13,16,25,26,27] Type 2 DM is known to affect the sympathetic and parasympathetic nervous system of the salivary glands, resulting in decreased salivary secretion, microangiopathy, dehydration, and hormonal changes, which may contribute to the decrease in the salivary flow rate.[13] However, few authors[28,29] were not able to establish significant difference in salivary flow rates between type 2 DM and healthy controls. We found a significant difference in the salivary pH between the type 2 DM patients and control (P < 0.01), which was similar to other studies.[16,20,27]
In accordance with previous studies,[28,30] we found significant differences (P < 0.05) in the buffering capacity between type 2 DM and control groups. This can also be attributed to the hormonal and metabolic changes in diabetic patients causing altered levels of salivary buffering systems. Results contrary to our study have been reported by Collin et al.[31]
In the present study, there was significant correlation (P < 0.01) between the salivary flow rate and salivary pH in the diabetics, and such correlation was not observed in the control group individuals. Even though type 2 DM patients had significant decrease in salivary flow rate in our study, it was observed that salivary flow rates were not as low as those in patients suffering from hyposalivation. This could be caused by increased fluid intake by diabetics due to polydipsia. Because buffering capacity is dependent on the pH levels, type 2 DM had salivary buffering capacity correlating with the salivary pH. Interestingly, it was observed that, in the control group, salivary pH levels were within the normal limits independent of the salivary flow rate. This could be due to reduced acidogenic flora in the oral cavity and increased salivary clearance activity maintaining normal pH levels.
Demmer et al. reported that, in patients with type 2 DM, the risk of periodontal disease is three times higher than that in the general population.[32] Similarly, we found that the type 2 DM patients had significantly poor periodontal status than the healthy controls. This is in accordance to previous studies.[7,33,34,35,36] It has been shown that DM causes alterations in the connective tissue metabolism by uncoupling the resorptive and formative processes, thus leading to increased levels of loss of periodontal attachment and bone loss.[37]
Among all the parameters tested in our study, only RCBG and UWSG showed significant positive correlation (P < 0.01) with the RPI scores in type 2 DM patients. None of the other salivary parameters studied correlated with the RPI scores, indicating that the level of glycemic control is an important determinant in being a risk factor for the development of gingivitis and periodontitis in type 2 DM.
Studies concerning the occurrence of caries in diabetic patients have yielded controversial results. In the present study, no significant difference was observed in the DMFT scores between the type 2 DM and controls, similar to earlier studies.[31,38] Lack of significant difference in the DMFT scores between the groups could be due to modification in the diet with reduced amounts of refined carbohydrate intake by the type 2 DM patients, thereby reducing the formation of an acidogenic environment. The fact that most of the patients who formed the study group belonged to the urban population and had unproblematic access to dental care could have also contributed to no significant differences in the mean DMFT scores between the type 2 DM and control groups. Our results are contrary to a few authors[6,16,25,34,39,40] who have reported that diabetics have slightly higher mean DMFT scores than the controls.
CONCLUSION
From our results, it can be concluded that the salivary glucose levels reflect the random blood glucose levels. Type 2 diabetics have significantly lower salivary flow rate, pH, and buffering capacity and present with advanced periodontal destruction than the healthy population. Limitation of our study would be the relatively smaller sample size. Further studies with larger sample size are warranted to substantiate the correlation between blood glucose and salivary glucose to devise saliva-based tests for diagnosing DM. Fasting salivary glucose estimation would also be an interesting area for further research.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Haslett C, Chilvers ER, Hunter JAA, Boon NA. Textbook on Principles and Practice of medicine. 18th edition. Churchill Livingstone; 1999. pp. 472–4. [Google Scholar]
- 2.Panda A, Venkatapathy R, Nirima O. Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2012;5:149–54. doi: 10.2147/DMSO.S32112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pradeepa R, Deepa R, Mohan V. Epidemiology of diabetes in India-current perspective and future projections. J Indian Med Assoc. 2002;100:144–8. [PubMed] [Google Scholar]
- 4.Spielmann N, Wong DT. Saliva: Diagnostics and therapeutic perspectives. Oral Dis. 2011;17:345–54. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sreedevi, Shashikanth MC, Shambulingappa P. Comparison of serum glucose and salivary glucose in diabetic patients. J Indian Acad Oral Med Radiol. 2008;20:9–13. [Google Scholar]
- 6.Bacic M, Ciglar I, Granic M, Plancak D, Sutalo J. Dental Status in a Group of Adult Diabetic Patients. Comm Dent Oral Epidemiol. 1989;17:313–6. doi: 10.1111/j.1600-0528.1989.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira Lima DL, Cardoso Neto AM, de Araújo KVC, Mendonça JS, Fernandes VO, Montenegro RM. Comparison of Oral Status Among Diabetic and Non-Diabetic Elders: A Pilot Study. Jacobs Journal of Dentistry and Research. 2014;2:1–6. [Google Scholar]
- 8.Sashikumar R, Kannan R. Salivary glucose levels and oral candidal carriage in type II diabetics. J Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:706–11. doi: 10.1016/j.tripleo.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 9.Jurysta C, Bulur N, Oguzhan B, Satman I, Yilmaz TM, Malaisse WJ, Sener A. Salivary Glucose Concentration and Excretion in Normal and Diabetic Subjects. J Biomed Biotechnol. 2009;2009:430426. doi: 10.1155/2009/430426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahadian H, Karbassi MH, Afkhami-Ardekani M, Haydaripoor Z, Sadrabad MJ, Kheirollahi K, et al. Comparison of the unstimulated whole salivary flow rate in Diabetic Type II patients with healthy individuals. Iran J Diabetes Obesity. 2014;6:93–7. [Google Scholar]
- 11.Lee HK, Choi SH, Won KC, Merchant AT, Song KB, Jeong SH, et al. The Effect of Intensive Oral Hygiene Care on Gingivitis and Periodontal Destruction in Type 2 Diabetic Patients. Yonsei Med J. 2009;50:529–36. doi: 10.3349/ymj.2009.50.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascarenhas P, Fatela B, Barahona I. Effect of Diabetes Mellitus Type 2 on Salivary Glucose – A Systematic Review and Meta Analysis of Observational Studies. Plos One. 2014;9:e101706. doi: 10.1371/journal.pone.0101706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasconcelos ACU, Soares MSM, Almeida PC, Soares T. Comparative study of salivary and blood glucose in Type 2 diabetic patients. J Oral Sci. 2010;52:293–8. doi: 10.2334/josnusd.52.293. [DOI] [PubMed] [Google Scholar]
- 14.Englander HR, Jeffay AI, Fuller IB, Chauncey HH. Glucose Concentrations in Blood Plasma and Parotid Saliva of Individuals with and without Diabetes Mellitus. J Dent Res. 1963;42:1246–9. doi: 10.1177/00220345630420052301. [DOI] [PubMed] [Google Scholar]
- 15.Mussavira S, Dharmalingam M, Omana Sukumaran B. Salivary glucose and antioxidant defense markers in type II diabetes mellitus. Turk J Med Sci. 2015;45:141–7. doi: 10.3906/sag-1306-116. [DOI] [PubMed] [Google Scholar]
- 16.Jawed M, Shahid SM, Qader SA, Azhar A. Dental caries in diabetes mellitus: Role of salivary flow rate and minerals. J Diabetes Complications. 2011;25:183–6. doi: 10.1016/j.jdiacomp.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Padmashree S, Jayalekshmi R. Correlation of salivary glucose, blood glucose and oral candidal carriage in the saliva of type 2 diabetics: A case-control study. Contemp Clin Dent. 2014;5:312–7. doi: 10.4103/0976-237X.137925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Sandhu SV, Bansal H, Sharma D. Comparison of Salivary and Serum Glucose Levels in Diabetic Patient. J Diabetes Sci Technol. 2015;9:91–6. doi: 10.1177/1932296814552673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadashetti V, Baad R, Malik N, Shivakumar KM, Vibhute N, Belgaumi U, et al. Glucose Level Estimation in Diabetes Mellitus By Saliva: A Bloodless Revolution. Rom J Intern Med. 2015;53:248–52. doi: 10.1515/rjim-2015-0032. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi A, Qureshi A, Qureshi H, Khan AA. Blood Glucose Level, Salivary pH and Oral Bacterial Count in Type 1 Diabetic Children. Infect Dis Pak. 2007;47:45–8. [Google Scholar]
- 21.Satish BN, Srikala P, Maharudrappa B, Awanti SM, Kumar P, Hugar D. Saliva: A tool in assessing glucose levels in Diabetes Mellitus. J Int Oral Health. 2014;6:114–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Belazi MA, Galli-Tsinopoulou A, Drakoulakos D, Fleva A, Papanayiotou PH. Salivary alterations in insulin dependent Diabetes Mellitus. Int J Pediatr Dent. 1998;8:29–3. doi: 10.1046/j.1365-263x.1998.00057.x. [DOI] [PubMed] [Google Scholar]
- 23.Campbell MJA. Glucose in the saliva of the non-diabetic and the diabetic patient. Arch Oral Biol. 1965;10:197–205. doi: 10.1016/0003-9969(65)90020-8. [DOI] [PubMed] [Google Scholar]
- 24.Lima Aragao MV, Oleivera JJ, Goncalves Cristina MM, Silva AL, Fernandes FR, Rosanne NMG. Salivary profile diabetic patients: Biological and Immunological evaluation. BMC Res Notes. 2016;9:103–9. doi: 10.1186/s13104-016-1881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaziri BP, Vahedi M, Mortazavi H, Abdollahzadeh S, Hajilooi M. Evaluation of Salivary Glucose, IgA and Flow Rate in Diabetic Patients: A Case-Control Study. J Dent. 2010;7(1):13–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Malicka B, Kaczmarek U, Skośkiewicz-Malinowska K. Prevalence of Xerostomia and the Salivary Flow Rate in Diabetic Patients. Adv Clin Exp Med. 2014;23:225–33. doi: 10.17219/acem/37067. [DOI] [PubMed] [Google Scholar]
- 27.Prathibha KM, Johnson P, Ganesh M, Subhashini AS. > Evaluation of Salivary Profile among Adult Type 2 Diabetes Mellitus Patients in South India. J Clin Diagn Res. 2013;7:1592–5. doi: 10.7860/JCDR/2013/5749.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meurman JH. Saliva in non-insulin-dependent diabetic patients and control subjects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:69–76. doi: 10.1016/s1079-2104(98)90152-4. [DOI] [PubMed] [Google Scholar]
- 29.Dodds MWJ, Dodd AP. Effects of glycemic control on saliva flow rates and protein composition in non-insulin-dependent diabetes mellitus. J Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:465–70. doi: 10.1016/s1079-2104(97)90147-5. [DOI] [PubMed] [Google Scholar]
- 30.Lima DC, Nakata GC, Balducci I, Almeida JD. Oral manifestations of Diabetes mellitus in complete denture wearers. J Prosthet Dent. 2008;99:60–5. doi: 10.1016/S0022-3913(08)60010-4. [DOI] [PubMed] [Google Scholar]
- 31.Collin HL. Caries in patients with non-insulin-dependent diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:680–5. doi: 10.1016/s1079-2104(98)90035-x. [DOI] [PubMed] [Google Scholar]
- 32.Demmer RT, Jacobs DR, Desvarieux M. Periodontal Disease and Incident Type 2 Diabetes. Diabetes Care. 2008;31:1373–9. doi: 10.2337/dc08-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanco JJA, Villar BB, Martinez EJ, Vallejo PS, Blanco FJA. Problemas bucodentales en pacientes con diabetes mellitus (I): Indice de placa y caries dental. Med Oral. 2003;8:97–109. [Google Scholar]
- 34.Sandberg GE, Sundberg HE, Fjellstrom CA, Wikblad KA. Type 2 diabetes and oral health: A comparison between diabetic and non-diabetic subjects. Diabetes Res Clin Pract. 2000;50:27–34. doi: 10.1016/s0168-8227(00)00159-5. [DOI] [PubMed] [Google Scholar]
- 35.Albrecht M, Banoczy J, Tamas G. Dental and oral symptoms of diabetes mellitus. Community Dent Oral Epidemiol. 1988;16:378–80. doi: 10.1111/j.1600-0528.1988.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 36.Kesavan R, Chaly PE, Reddy VC, Mary AV. Periodontal status among type II diabetic and nondiabetic individuals in Chennai, India: A comparative study. J Indian Assoc Pub Health Dent. 2015;13:393–8. [Google Scholar]
- 37.Ryan ME, Carnu O, Kamer A. The Influence of diabetes on Periodontal tissues. JADA. 2003;134:34S–40S. doi: 10.14219/jada.archive.2003.0370. [DOI] [PubMed] [Google Scholar]
- 38.Al Attas SA, Oda SA. Caries experience and selected caries-risk factors among a group of adult diabetics. Saudi Dent J. 2008;20:129–39. [Google Scholar]
- 39.Sukminingrum N, Ishak I, Masudi SA, Alam MK. Comparison of Decayed, Missing or Filled Teeth (DMFT) Indexes between Diabetic and Non-Diabetic Patients. Int Med J. 2013;20:443–5. [Google Scholar]
- 40.Arul A, Sri Kennath J, Sanjay R, Peramachi P. Evaluation of correlation between Salivary pH and prevalence of Dental Caries in subjects with and without Diabetes Mellitus. Research Journal of Recent Sciences, 2014;3:224–6. [Google Scholar]


