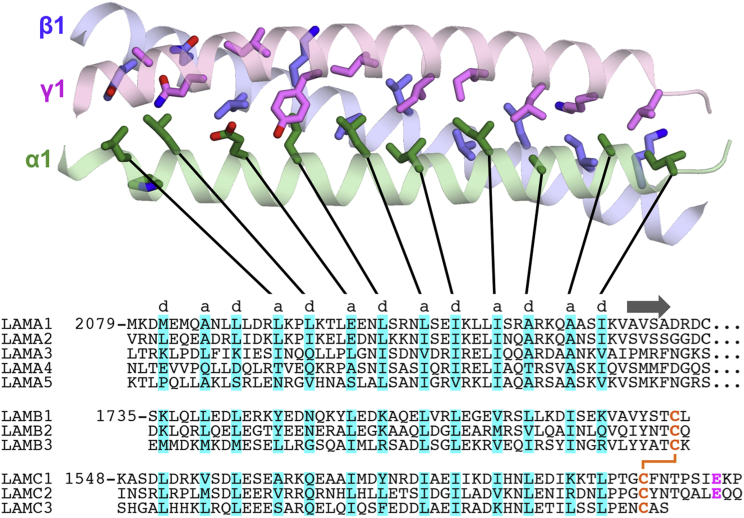
Figure 2.
Structure and Sequence Conservation of the Coiled Coil
The N termini of the three helices (α1 chain, green; β1 chain, blue; γ1 chain, magenta) are on the left. The side chains of residues in the a and d positions of the heptad repeats (Cohen and Parry, 1990) are shown as sticks. Below the structure is an alignment of all mouse laminin chains with the a and d positions highlighted in cyan. The first β strand of the α1 LG1 domain is indicated by an arrow. The β1-γ1 inter-chain disulfide bond and the critical glutamic acid in the γ1 chain are in orange and magenta, respectively.