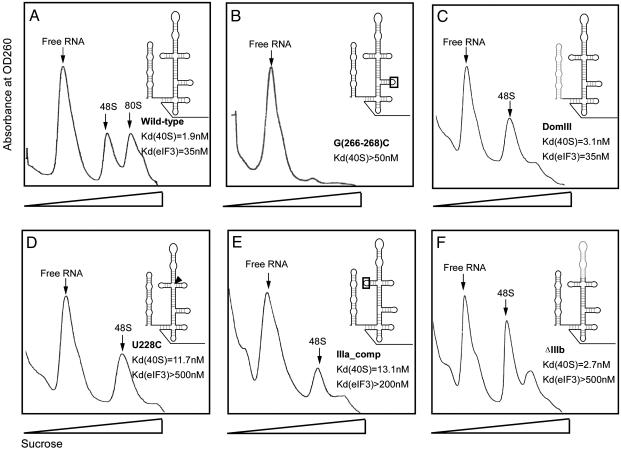
Fig. 2.
Sucrose density gradient analysis of translation complexes bound by wild-type and mutant forms of the HCV IRES. Affinity-tagged IRES RNAs were incubated in HeLa cell extract at a concentration of extract yielding half-maximal binding of wild-type IRES. Plots of absorbance at 260 nm versus sucrose density are shown; peaks corresponding to free RNA, 48S, and 80S are indicated (verified by denaturing PAGE and electrospray mass spectrometry). (A) Wild-type IRES. (B–F) Translation-defective IRES constructs containing the following mutations and translation complex binding defects relative to the wild-type HCV IRES (12, 23). (B) G(266–268)C mutation in the IIId loop, >25-fold-reduced 40S binding affinity. (C) DomIII, no change in 40S or eIF3 binding affinities. (D) U228C, >15-fold-reduced eIF3 binding affinity. (E) IIIa_Comp, >6-fold-reduced eIF3 binding affinity. (F) ΔIIIb, >15-fold-reduced eIF3 binding affinity.