Figure 5.
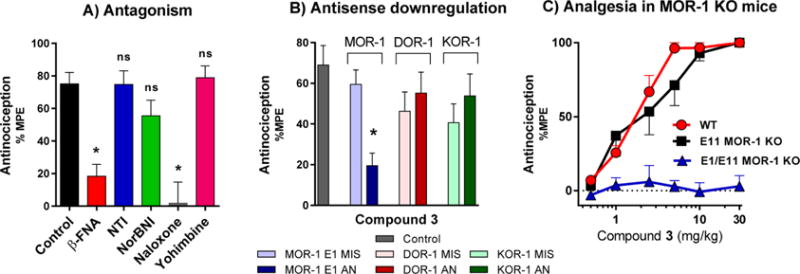
Pharmacological and genetic reversal of antinociception of 3. (A) Reversal of antinociception by selective antagonists. Groups of CD1 mice (n = 10) received 3 (1.5 mg/kg sc) and the indicated antagonist. β-Funaltrexamine (β-FNA; 40 mg/kg sc) and norbinaltorphimine (norBNI; 10 mg/kg sc) were administered 24 h before agonist testing. Naltrindole (NTI; 0.5 mg/kg sc), naloxone (1 mg/kg), and yohimbine (10 mg/kg) were administered 15 min before 3. All antinociception testing was performed 15 min after the administration of 3. Similar results were observed in two independent replications. 3 antinociception is insensitive to NTI, norBNI, and yohimbine, whereas antinociception is antagonized by β-FNA and naloxone (two-way ANOVA followed by Bonferroni post hoc comparisons test, p < 0.05). All values are expressed as the mean ± SEM. (B) Antisense oligodeoxynucleotide injection: Groups of mice (n = 15) received the stated antisense (5–10 μg) or mismatch (5–10 μg) oligodeoxynucleotide icv under light isoflurane anesthesia on days 1, 3, and 5. Tail flick antinociception was tested on day 6. Control groups received no injection prior to testing. On test day, mice received 3 (1.5 mg/kg, sc). All experiments were performed 3 times with similar results observed with each determination. Analgesic response of 3 was only affected in mu receptor downregulated mice (MOR-1 AN) (one-way ANOVA followed by Bonferroni post hoc comparisons test). *Significantly different from control (p < 0.05). Data for agonist controls are shown in the Figure S6 and sequences of AN and MIS oligos are shown in Table 4. (C) Antinociception of 3 in wild-type, exon 11 KO, and exon 1/exon 11 double KO C57 mice. Two independent determinations of the cumulative dose–response curves were performed on groups of mice (n = 5) for antinociception in the tail flick assay with 3 given subcutaneously. Compound 3 displayed similar antinociceptive effects in wild-type (ED50 = 0.83 mg/kg (0.37–1.9)) and exon 11 KO mice (ED50 = 1.4 mg/kg (0.34–5.8)), however, no antinociception was observed in exon 1/exon 11 double KO mice, suggesting that the antinociceptive effect of 3 is mediated by the E1MOR-1 variants.
