Scheme 1. Synthesis of Mitragynine Derivatives 2–12a.
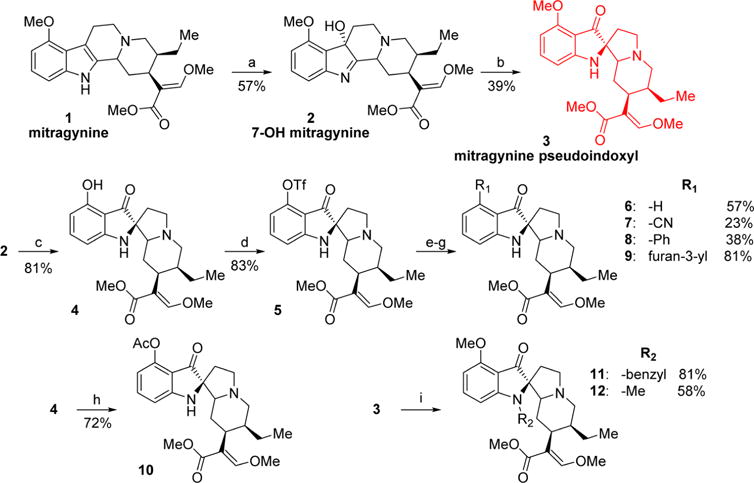
aReagents and conditions: (a) PIFA, H2O, acetonitrile, 0 °C, 1 h; (b) Zn(OTf)2, toluene, 110 °C, 2 h; (c) AlCl3, EtSH, DCM, 0 °C, 5 h; (d) Tf2O, pyridine, DCM, −40 °C, 1 h; (e, yielding 6) Pd(OAc)2, dppp, HCOOH, DMF, 60 °C, 1 h; (f, yielding 7) Zn(CN)2, Pd(PPh3)4, DMF, 80 °C, 2 h; (g, yielding 8 and 9) phenylboronic acid (8) or 3-furanylboronic acid (9), Pd(PPh3)4, K2CO3, MeOH, toluene, 80 °C, 2 h; (h) Ac2O, pyridine, rt, 1 h; (i, yielding 11 and 12) benzyl bromide (11) or iodomethane (12), NaH, acetonitrile, rt, 2 h.
