Figure 1.
Histone Serine ADPr Is Dependent on HPF1
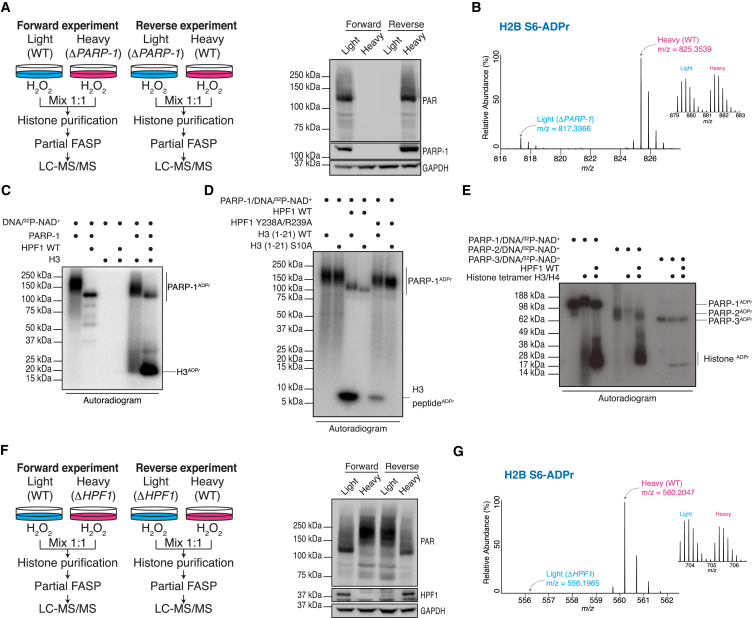
(A) SILAC strategy to quantify core histone ADPr marks after DNA damage in WT and ΔPARP-1 U2OS cells (left) and a representative western blot of total protein poly-ADP-ribosylation prior to mixing light and heavy lysates from each SILAC experiment (right). Anti-GAPDH was used as a loading control.
(B) MS1 of a PARP-1-sensitive modified H2B peptide. The heavy peptide was derived from WT cells, and the light peptide was derived from ΔPARP-1 cells (both stimulated with H2O2). The inset (right) shows an ∼1:1 ratio (heavy/light) of a non-ADP-ribosylated peptide from the same experiment.
(C) Autoradiogram shows ADP-ribosylation of recombinant H3 by PARP-1 in the presence of HPF1.
(D) Autoradiogram shows ADP-ribosylation of two synthetic peptide variants corresponding to amino acids 1–21 of human H3.
(E) Autoradiogram shows histone tetramer ADP-ribosylation by PARP-1, PARP-2, or PARP-3 in the presence of HPF1.
(F) SILAC strategy to quantify core histone ADPr marks upon DNA damage in WT and ΔHPF1 U2OS cells (left) and a representative western blot of total protein poly-ADP-ribosylation levels prior to mixing light and heavy lysates from each SILAC experiment (right). Anti-GAPDH was used as a loading control.
(G) MS1 of an HPF1-sensitive H2B-modified peptide. The heavy peptide was derived from the WT cells, and the light peptide (very low intensity) was derived from ΔHPF1 cells (both were stimulated with H2O2). The inset (right) shows an ∼1:1 ratio (heavy/light) of a non-ADP-ribosylated peptide from the same experiment.
See also Figure S1.