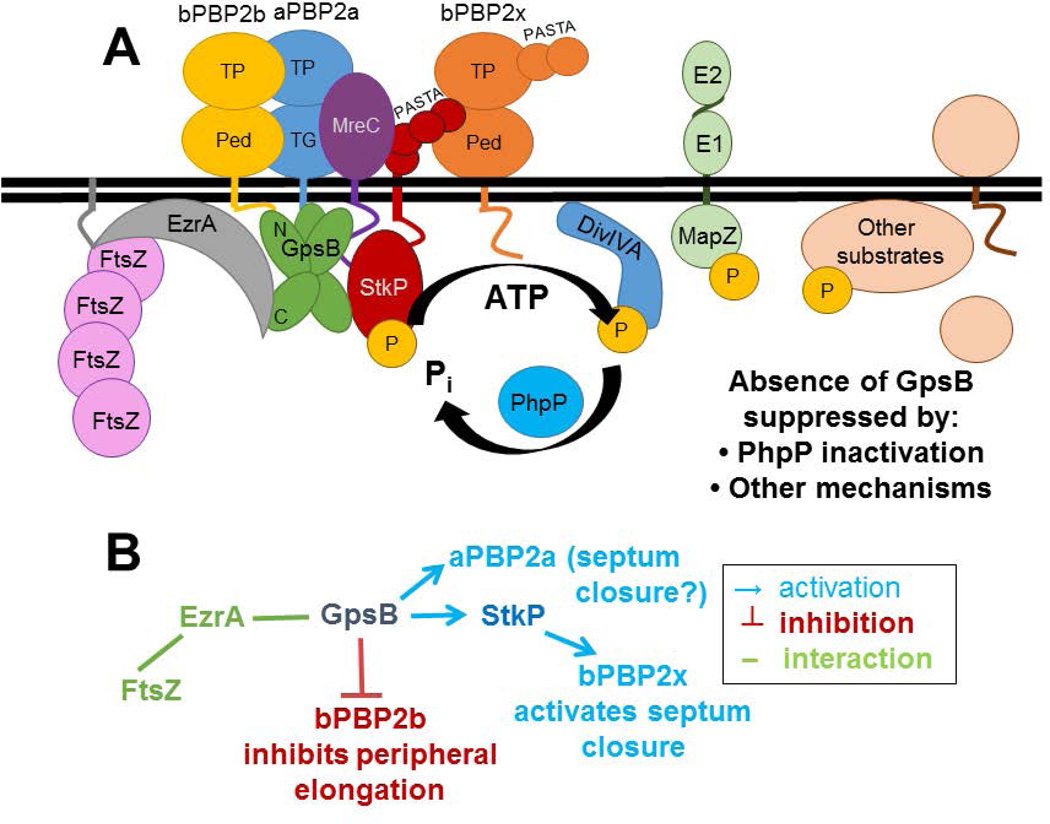
Fig. 10.
Model of GpsB interactions and coordination of septal and peripheral PG synthesis in Spn strain D39. A) Complexes containing EzrA, which binds to FtsZ, and GpsB link FtsZ-divisome dynamics (which are not shown) with GpsB regulation of downstream functions. Wild-type levels of GpsB mediate the normal protein phosphorylation cycle by StkP kinase and PhpP phosphatase of numerous division proteins, including DivIVA, MapZ(LocZ), whose extracellular E1 and E2 domains are labeled, and other proteins. Septal and peripheral PG synthesis are coordinated by GpsB complexed with aPBP2a, bPBP2b, MreC, and StkP, which interacts with bPBP2x. bPBP2x and possibly aPBP2a catalyze septal ring closure, whereas bPBP2b and MreC catalyze peripheral PG synthesis. Deletion of gpsB is lethal and can be suppressed by non-polar mutations that inactivate the PhpP phosphatase, thereby implicating maintenance of protein phosphorylation levels as an important regulatory function of GpsB; however, the critical phosphorylated protein(s) remain to be determined. B) Genetic scheme of PBP activation by GpsB that can account for the enlarged, elongated cells with unconstricted septa caused by GpsB depletion. According to this scheme, which is based on phenotypes, genetic relationships, microscopy, and interaction maps, GpsB positively regulates septum closure by activating aPBP2a directly and bPBP2x indirectly, via an interaction between GpsB and StkP, whereas GpsB directly or indirectly inhibits bPBP2b/MreC and peripheral PG elongation. See text for additional details.