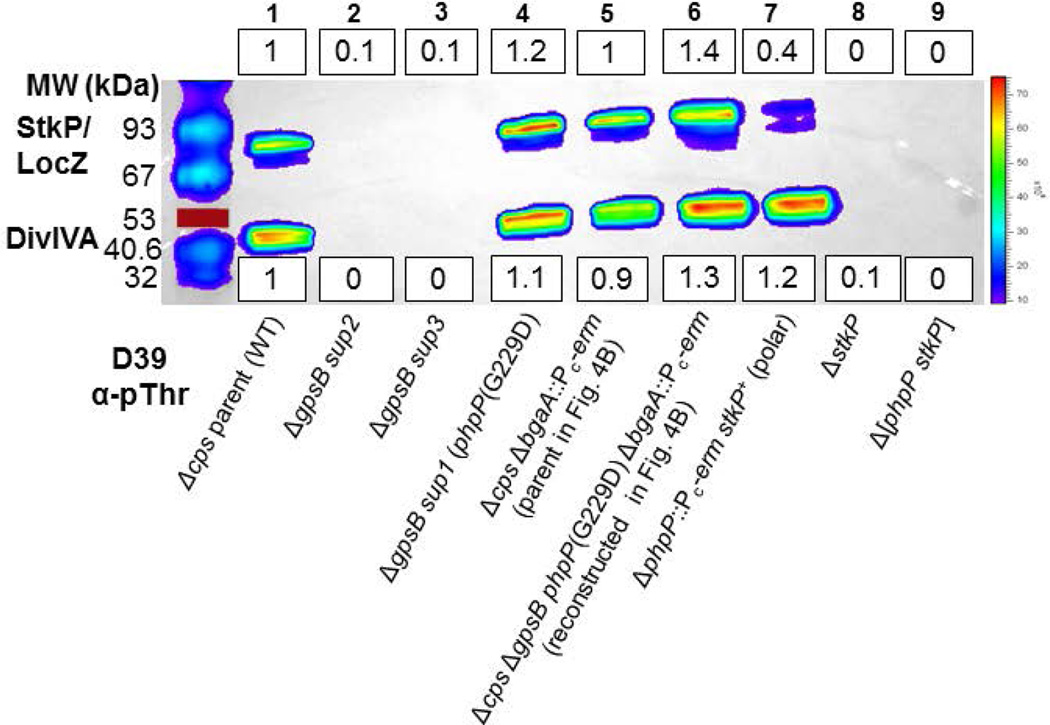
Fig. 3.
phpP(G229D) restores wild-type levels of protein phosphorylation to D39 Δcps ΔgpsB mutants in originally isolated and reconstructed suppressor strains. A representative Western blot of phosphorylated proteins was performed and quantitated as described in Figure 2 and Experimental procedures. Mean relative values (±SEM) of band intensities are compiled for all experiments in Table S7. Strains were harvested at OD620 ≈ 0.4. Lane 1, wild-type parent D39 Δcps (IU1945); lane 2, D39 Δcps ΔgpsB Δ[spd_1026–spd_1037] Ω[spd_0889–spd_1026] (IU5845, sup2) (Table 2, line 2); lane 3, D39 Δcps ΔgpsB Δ[spd_1026–spd_1037] Ω[spd_0889–spd_1026] (IU6441, sup3); (Table 2, line 3); lane 4, D39 Δcps ΔgpsB phpP(G229D) (IU6442, sup1) (Table 2, line 1); Lane 5, D39 Δcps ΔbgaA::Pc-erm (E46) (strain used in reconstruction; Fig. 4B); lane 6, D39 Δcps ΔbgaA::Pc-erm ΔgpsB phpP(G229D) (IU11221) (reconstructed suppressor; Fig. 4B); lane 7, D39 Δcps ΔphpP-Pc-erm (IU11442) (polar mutant with reduced StkP expression); lane 8, D39 Δcps ΔstkP (IU11460) control; and lane 9, D39 Δcps Δ[phpP-stkP] (IU11462) control. The experiment was performed twice independently with similar results. The red line marks a colored 53 kDa standard that did not transfer.