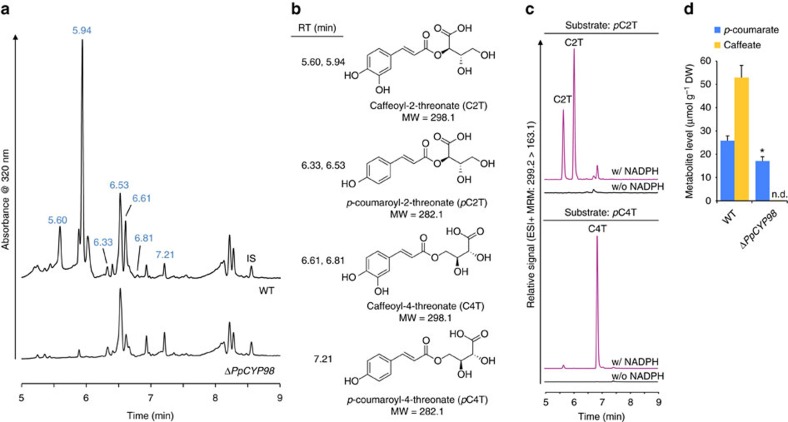
Figure 3. PpCYP98 is a phenolic ring meta-hydroxylase and uses esters of threonic acid as substrates.
(a) Ultraviolet chromatogram showing the absence of major peaks in the ΔPpCYP98 mutant gametophore crude extract. IS, internal standard (morin). (b) Names and structures of molecules at the indicated retention times (RT). (c) PpCYP98-dependent conversion of p-coumaroyl-2-threonate (pC2T) and p-coumaroyl-4-threonate (pC4T) esters into corresponding caffeoyl threonate esters (C2T and C4T). Control reactions without NADPH were concurrently analysed. Molecules were detected using dedicated multiple reaction monitoring (MRM) methods. Note that two caffeoyl-2-threonate isomers are produced from the two p-coumaroyl-2-threonate isomers present in the synthetic substrate, shown in Supplementary Fig. 6. (d) Acid hydrolysis of crude extracts demonstrates the total absence of caffeate in gametophores of the ΔPpCYP98 mutants. Results are the mean+standard error from three independent biological samples for WT and three independent mutant lines. Asterisk indicates a significant difference between mutants and WT (P-value=0.037; two-tailed Student's t-test for samples of unequal variance).