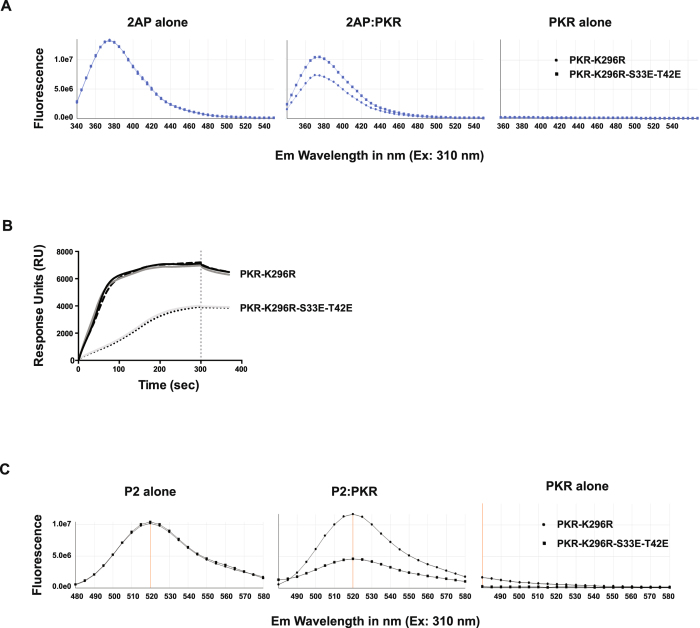
Figure 4. Phosphor-S33 and -T42 affect the state of the PKR kinase domain.
(A) Measures of the comparative in vitro binding of 2-aminopurine nitrate (2AP) to the indicated recombinant PKR protein by assessing changed fluorescence from the molecule. The graphs show that the separate PKR constructs differently diminished 2AP-fluorescence at a ratio of 1:2 of 2AP to PKR. The consequence of a broader range of concentrations of PKR and of alternative PKR constructs are shown in Supplementary Figure S2. (B) Absorption resonance curves for ligand coupling of the indicated recombinant PKR proteins, demonstrating that the phosphor-residues generate distinct profiles. SPR experiments were carried out on a ProteOn XPR36 (Bio-rad Labs) using an HTE chip for His-tagged proteins and TBS as the running buffer. PKR proteins were immobilized to the nickel-activated chip via their His-tags after dilution to 50 μg/ml. Data shown are technical replicates from representative duplicate experiments and show the Response Units (RU) measured from the surface of the chip over the course of the sunitinib coupling (0 to 300 sec) to the washing step (300 sec and beyond), indicated by the vertical dashed line on the graph. Surface plasmon resonance (SPR) sensorgrams of the experiment between the PKR and sunitinib are shown in Supplementary Figure S3. (C) Measures of the comparative binding of a FITC-tagged peptide (P2) to the indicated PKR constructs by assessing fluorescence. The consequence of a broader range of concentrations of PKR and of alternative PKR constructs for FITC-P2 fluorescence is shown in Supplementary Figure S4.