Figure 5. Modelling the interaction between RBM1 and the kinase domain.
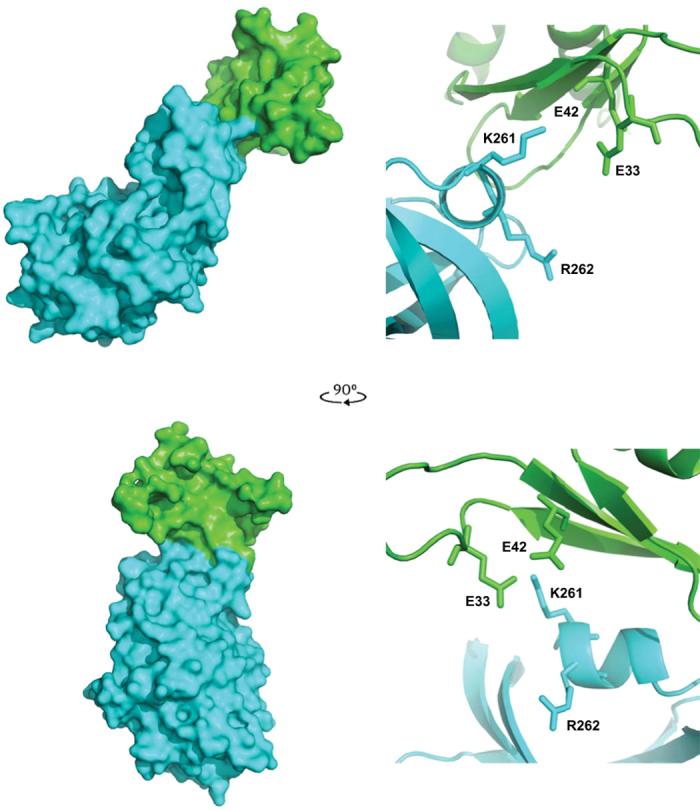
The predicted association between the PKR kinase domain (cerulean) in complex with the RBM1 (green) shown as a space-filling model (on left). Two perspectives of the complex are shown at right angles to each other. The RBM1 is predicted to associate with the N-lobe of the kinase domain, which faces the viewer in the lower perspective. Details of the docking orientation of the RBM1 with the kinase domain of PKR are shown as ribbon diagrams (on the right) with the side chains of the E33 and E42 as phosphor-mimetic mutations of S33 and T42, and putative associating K261 and R262 residues in the α0-helix on the kinase domain indicated. The orientation of the protein-docking interface shown as a ribbon diagram (on right) replicates that shown in the space-filling diagram of the complex (on the left).
