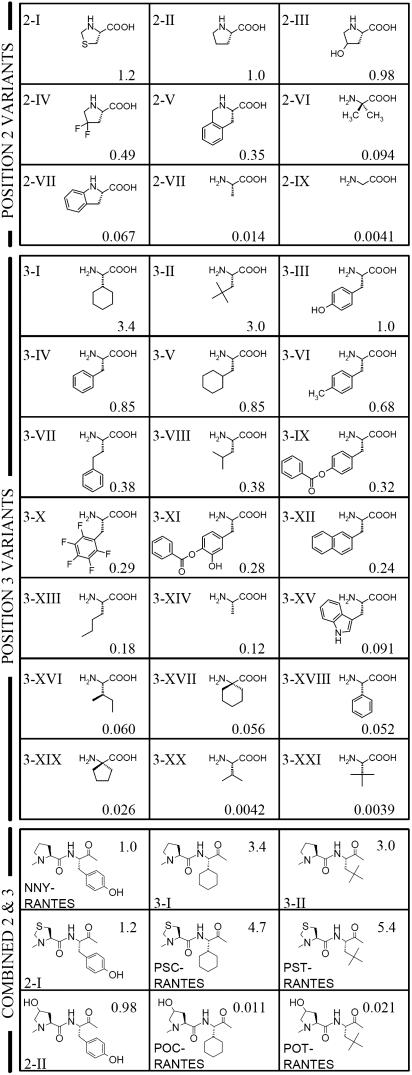
Fig. 2.
Second and third rounds of optimization; structure and anti-HIV activity of NNY-RANTES analogs. Activity indices (two significant figures) are expressed as the potency (IC50) of a given molecule relative to that of NNY-RANTES measured in the same experiment and are shown to the right of each structure. For the second cycle of optimization, Roman numerals are used to denote structures of amino acid substituents used in NNY-RANTES analogs, which are displayed in order of decreasing potency (see below). For the third cycle of optimization (combined substitution at positions 2 and 3), structures corresponding to positions 2 and 3 of NNY-RANTES are shown. Position 2 variants: 2-I; thiazolidine-4-carboxylic acid, 2-II; proline, 2-III; 4-hydroxyproline, 2-IV; 4,4-difluoroproline, 2-V; 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, 2-VI; aminoisobutyric acid, 2-VII; indoline-2-carboxylic acid, 2-VIII; alanine, 2-IX; glycine. Position 3 variants: 3-I; cyclohexylglycine, 3-II; t-butylalanine, 3-III; tyrosine, 3-IV; phenylalanine, 3-V; cyclohexylalanine, 3-VI; 4-methylphenylalanine, 3-VII; homophenylalanine, 3-VIII; leucine, 3-IX; p-benzoyl-tyrosine, 3-X; pentafluorophenylalanine, 3-XI; 3-hydroxy, 4-benzoyl-tyrosine, 3-XII; 2-naphthylalanine, 3-XIII; norleucine, 3-XIV; alanine, 3-XV; tryptophan, 3-XVI; isoleucine, 3-XVII; 1-amino-1-cyclohexanecarboxylic acid, 3-XVIII; phenylglycine, 3-XIX; and 1-amino-1-cyclopentanecarboxylic acid, 3-XX; and valine, 3-XXI; t-leucine.