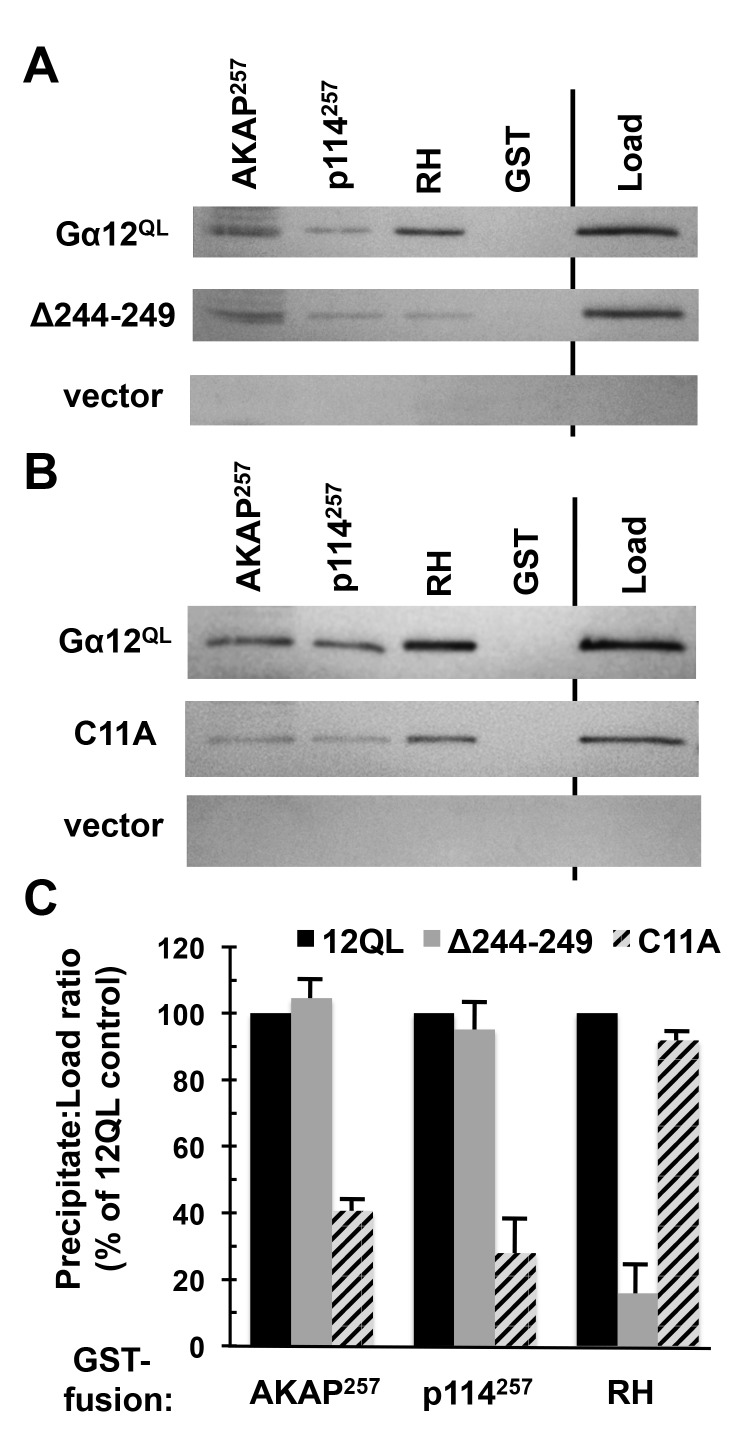
Figure 3.
Gα12 binding to AKAP-Lbc and p114RhoGEF is distinct from RH-RhoGEF interaction. (A, B) Results of protein interaction experiments using mutant forms of myc-tagged, constitutively active Gα12 (Gα12QL). For both panels, Sepharose-immobilized AKAP-Lbc and p114RhoGEF constructs utilized in Figure 2B were tested, alongside the immobilized RH domain of LARG (RH), for ability to co-precipitate Gα12 variants from HEK293 cell extracts as described in Methods. Load samples were set aside prior to the precipitation step. Representative results are shown. (C) Immunoblot bands for Gα12 mutants were quantified using ImageJ, and for each sample the precipitate:load ratio was calculated and presented as a % of the precipitate:load ratio for non-mutated, constitutively active Gα12. Results shown are from three or more independent experiments, with graphs indicating mean ± standard error of the mean (s.e.m.).