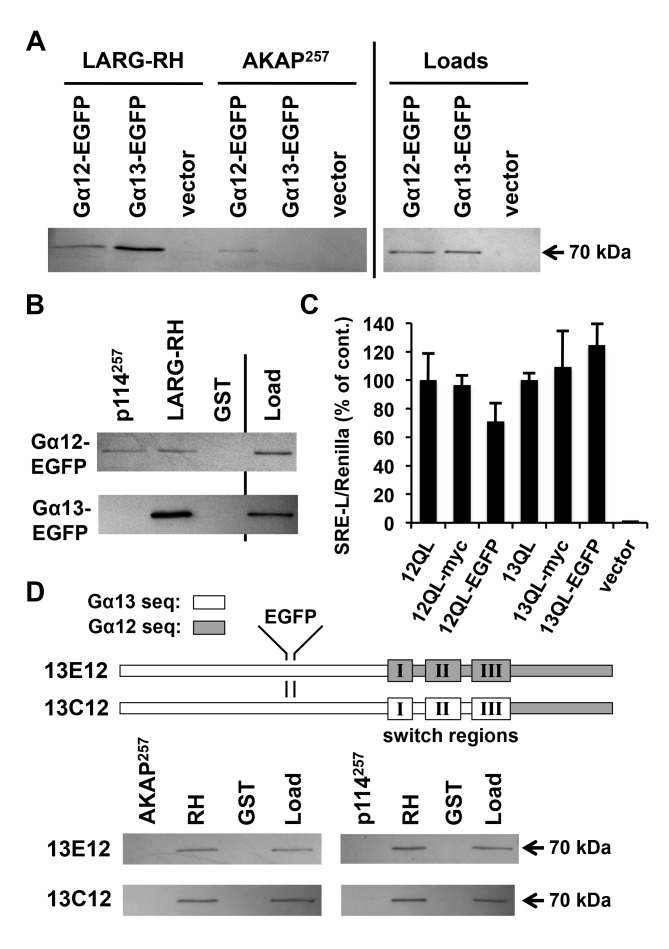
Figure 4.
AKAP-Lbc and p114RhoGEF are selective for Gα12 binding. (A, B) Binding of EGFP-tagged G12/13 α subunits to RhoGEF domains. GST-fusions of the 257-amino acid AKAP-Lbc and p114RhoGEF regions used in Figure 2B, as well as the RH domain of LARG, were examined for ability to co-precipitate EGFP-tagged, constitutively active Gα12 and Gα13. Unmodified GST was tested as a negative control for α subunit co-precipitation. Shown are immunoblot analyses of precipitates and loads using an anti-EGFP antibody. All bands in these images co-migrated approximately with a 70 kDa protein standard (PageRuler; ThermoFisher). (C) Serum response element (SRE)-luciferase activation by epitope-tagged forms of constitutively active Gα12 and Gα13. Reporter constructs used were SRE-L, encoding firefly luciferase under control of a SRE-containing promoter, and pRL-TK, encoding Renilla luciferase governed by a thymidine kinase promoter. HEK293 cells grown in 12-well plates were transfected with SRE-L (0.2 µg) and pRL-TK (0.02 µg), plus plasmids encoding Gα12 or Gα13 as untagged, internally myc-tagged, or internally EGFP-tagged forms (0.1 µg). All constructs harbored activating Gln-to-Leu mutations in the switch II region, Q229L for Gα12 and Q226L for Gα13. Cells were harvested approximately 48 h post-transfection and assayed for luciferase activity as described in Methods. Firefly activity normalized for Renilla activity is shown for each sample (Y-axis) and presented as % of the untagged Gα12 or Gα13 response. Data presented are the mean of three independent experiments, with error bars indicating range. (D) Binding of Gα13/Gα12 chimeras to regions of RhoGEFs. A schematic of these chimeras, previously reported [47] and provided by Barry Kreutz (Univ. of Illinois at Chicago), is shown with an EGFP tag positioned as described in Methods. Both constructs harbor activating (QL) mutations in the switch II region. Lysates of HEK293 cells transfected with these tagged chimeras were subjected to co-precipitations by RhoGEF domains as described in (A) and (B), above. Immunoblot results using anti-EGFP antibody are shown, and are representative of three independent experiments.