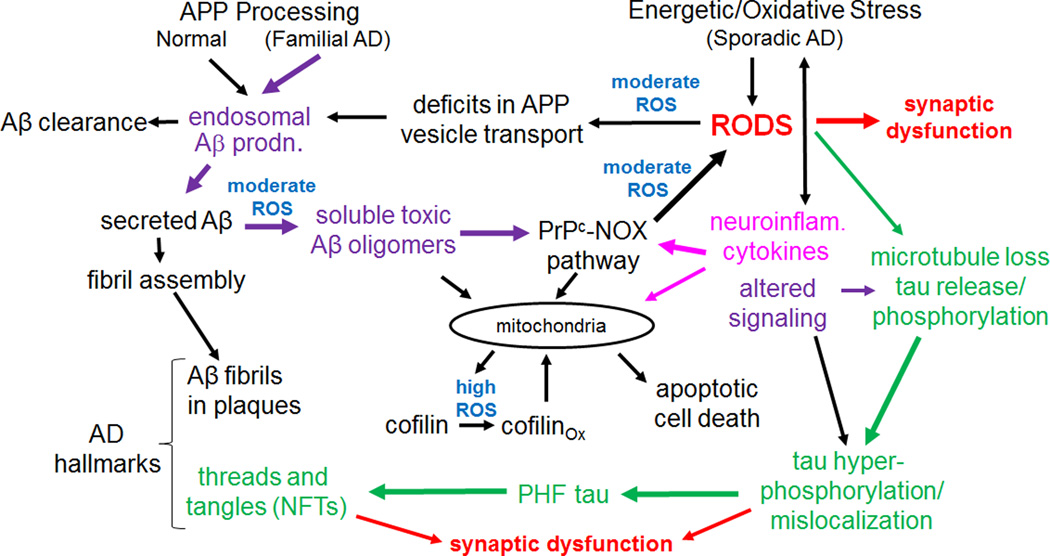
Figure 8. A hypothetical model in which rods play a central role in mediating AD progression.
Intermediates or initiators for the three major hypotheses for AD are shown in color: purple for the amyloid hypothesis, green for the tauopathy hypothesis and fuscia for the neuroinflammatory/cytokine hypothesis. Sites where reactive oxygen species are thought to be directly involved are in blue. In cognitively normal individuals, Aβ produced in endosomes is cleared or it may form fibrils and be sequestered into extracellular plaques in the brain. Although found in familial and sporadic AD, plaques may also be found in cognitively normal subjects and thus by themselves do not cause dementia. Changes in the form of soluble Aβ, arising from oxidation or other modifications, produce a more rod-inducing and synaptotoxic form. Other initiators of sporadic AD, such as neuroinflammatory cytokines, initiate a moderate ROS response that leads to rods and greater amounts of Aβ production in a feed-forward pathway. We hypothesize that rod-induced microtubule disruption releases tau for phosphorylation and initiation of PHF-tau formation, although this could be directly mediated by altered signaling. Mitochondrial dysfunction resulting from many possible sources eventually produces high ROS levels that contribute to cofilin oxidation (two internal disulfide bonds) that target it to mitochondria and cause release of cytochrome c, initiating apoptosis. Since rods are reversible, cofilin-induced apoptosis may gradually increase during cycles of rod reversal and acute high ROS levels.