Figure 2.
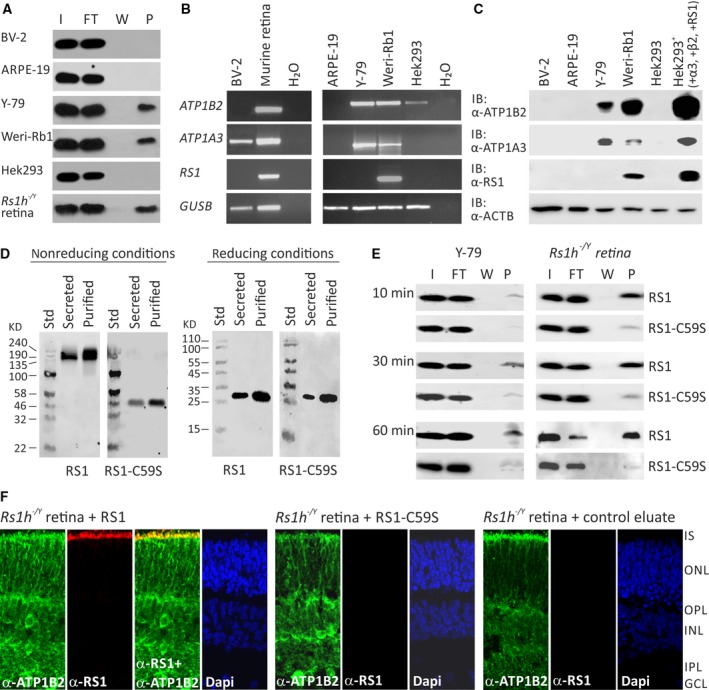
Binding of RS1 variants to retinal cells (A) Binding of retinoschisin to cultured retinal cell lines ARPE‐19, Y‐79, Weri‐Rb1 and BV‐2: Cells were incubated for 60 min. with retinoschisin containing supernatant (I, input) of cells stably transfected with a retinoschisin expression vector. Subsequently, cells were centrifuged and supernatant (FT, flowthrough) was discarded. After further washing steps (last supernatant, W), cells were pelleted (pellet, P). Fractions were subjected to Western blot analyses using an antiretinoschisin antibody. Retinoschisin binding to Hek293 cells and murine Rs1h −/Y retinal membranes served as negative and positive controls, respectively. (B) RT‐PCR analysis of ATP1B2, ATP1A3 and RS1 gene expression in cell lines derived from murine microglia (BV‐2), human retinal pigment epithelium (ARPE‐19), human retinoblastoma (Y‐79 and Weri‐Rb1) and human embryonic kidney (Hek293). GUSB gene expression was assessed as control for RNA integrity. (C) Cell lysates from BV‐2, ARPE‐19, Y‐79, Weri‐Rb1 and Hek293 were subjected to Western blot analyses using antibodies against ATP1B2, ATP1A3 and retinoschisin. Hek293+ cells served as positive control. The ACTB immunoblot was performed as loading control. (D) Oligomerization of RS1 variants (non‐mutant retinoschisin and RS1‐C59S) before and after purification. About 48 hrs after transfection of Hek293 cells with expression constructs for N‐terminally Myc‐tagged RS1 variants, the cell culture medium (supernatant) was harvested and Myc‐tagged proteins were purified from the supernatant. Aliquots of supernatant and purified RS1 fractions were subjected to SDS‐PAGE under non‐reducing and reducing conditions, followed by Western blot analyses using an antiretinoschisin antibody. (E) Binding of RS1 variants to retinal cells. Y‐79 cells and murine Rs1h −/Y retinal explants were incubated with purified RS1 variants (I, input) for 10, 30 and 60 min. Cells were centrifuged and supernatant (FT, flowthrough) was discarded. After several washing steps (last supernatant, W), cells were pelleted (pellet, P). Fractions were subjected to Western blot analyses using an antiretinoschisin antibody. (F) Localization of recombinant RS1 variants on retinal membranes. Rs1h −/Y retinal explants (P10) were incubated for 30 min. with retinoschisin, RS1‐C59S or control protein, the latter purified from supernatant of empty expression vector‐transfected cells. After washing and embedding, cryosections of these explants were subjected to immunohistochemical analyses using antibodies against ATP1B2 and retinoschisin. 4′,6‐Diamidino‐2‐phenylindol (DAPI) staining shows the nuclei of the different retinal layers. IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
