ABSTRACT
The maintenance of viable and functional pancreatic islets is crucial for successful islet transplantation from brain-dead donors. To overcome islet quality loss during culture, some studies have co-cultured islets with mesenchymal stem/stromal cells (MSC). However, it is still uncertain if MSC-secreted factors are enough to improve islet quality or if a physical contact between MSCs and islets is needed. Therefore, we performed a systematic review and meta-analysis to clarify the effect of different culture contact systems of islets with MSCs on viability and insulin secretion outcomes. Pubmed and Embase were searched. Twenty studies fulfilled the eligibility criteria and were included in the qualitative synthesis and/or meta-analysis. For both outcomes, pooled weighted mean differences (WMD) between islet cultured alone (control group) and the co-culture condition were calculated. Viability mean was higher in islets co-cultured with MSCs compared with islet cultured alone [WMD = 18.08 (95% CI 12.59–23.57)]. The improvement in viability was higher in islets co-cultured in indirect or mixed contact with MSCs than in direct physical contact (P <0.001). Moreover, the mean of insulin stimulation index (ISI) was higher in islets from co-culture condition compared with islet cultured alone [WMD = 0.83 (95% CI 0.54–1.13)], independently of contact system. Results from the studies that were analyzed only qualitatively are in accordance with meta-analysis data. Co-culture of islets with MSCs has the potential for protecting islets from injury during culture period. Moreover, culture time appears to influence the beneficial effect of different methods of co-culture on viability and function of islets.
KEYWORDS: co-culture, mesenchymal stem/stromal cells, Pancreatic islets, systematic review and meta-analysis, viability insulin secretion
Introduction
Type 1 diabetes mellitus (T1DM) is responsible for 5- to 10% of all cases of diabetes, and results from a cellular-mediated autoimmune destruction of pancreatic β cells, which renders individuals insulin-dependent for life.1 This disease is associated with chronic complications that lead to high morbidity and mortality rates in young adults of productive age.1,2 Intensive insulin therapy reduces the onset and progression of chronic diabetic complications, but is associated with an increased risk of severe hypoglycemia.2,3 In this context, allogeneic pancreatic islet transplantation offers a minimally invasive option for β cell replacement in T1DM patients that suffer from hypoglycemia unawareness with frequent episodes of severe hypoglycemia and marked glycemic lability.4-6
Although improvement in glycemic control and hypoglycemia awareness are commonly achieved in T1DM patients transplanted with a sub-optimal islet mass,6,7 the ultimate outcome of insulin independence usually requires multiple donors, limiting the number of patients benefiting from this therapy.8 It occurs because only a part of the total islet mass from a single brain-death donor can be successfully extract from the pancreas by the present isolation protocols.9,10
During isolation process islets suffer several insults, including oxidative, hypoxic and inflammatory stresses, that continuous to happen during the pre-transplant culture period. Other specific insults of this period are shortage of nutrients factors, pro-inflammatory molecules released by the islet themselves, and harmful enzymes released by acinar tissue.11-13 The combination of these factors seem to be responsible, at least in part, for the undesired loss of a considerable amount of viable islet mass that is observed right before transplantation, leading to a reduced chance of insulin independence achievement after single donor islet transplantation.14-18 Therefore, substantial research has been directed toward maintaining islet viability and function to improve islet transplantation success.11,19-24
An emerging strategy to improve islet viability and function and, thus, graft survival, involves co-culture of islets with mesenchymal stem/stromal cells (MSCs).25-31 MSCs exist in almost all tissues, residing mostly close to blood vessels. They have the capacity of self-renewal and the potential to differentiate into different cell types, such as adipocytes, chondrocytes, osteoblasts, myocytes and neurons.32,33 These cells have attracted significant attention in recent years due to their potent immunomodulatory properties activated by the surrounding microenvironment, where abundant inflammatory factors are released from immune cells.25,33 In addition, MSCs have been shown to secrete several paracrine molecules, which mediate trophic effects on neighboring cells, enhancing viability and function of islets during in vitro culture.26,27,31,33,34 However, studies are still inconclusive to define if MSC-secreted mediators are enough to improve islet survival and function26,27,35 or if a physical contact between MSCs and islets is also needed.30,31,36 Therefore, from our knowledge, this is the first systematic review and meta-analysis performed to evaluate the effect of indirect or direct co-culture of pancreatic islets with MSCs on islet viability and function.
Results
Literature search and quality of the eligible studies
Fig. 1 is a flow diagram showing the strategy used to identify and select studies for inclusion in this systematic review and meta-analysis. All studies that reported co-culture of pancreatic islets with MSCs and evaluated markers related to islet viability and/or function were selected for inclusion. A total of 3992 possibly relevant citations were retrieved by searching the electronic databases and one was found through manual search of selected articles. After duplicates removal, 3379 articles were screened based on title and abstracts, with 3331 of them being excluded during this review. Forty-eight articles remained to complete the data analysis. Nevertheless, after cautious analysis of the full texts, another 28 studies were excluded due to missing information, ineligible study designs, publication in Chinese or because other cell types were evaluated. Twenty studies fulfilled the eligibility criteria and were included in the systematic review and/or meta-analysis. Among these articles, only 12 studies26-30,33,35-40 had complete data of interest for at least one outcome [viability and/or insulin secretion index (ISI)] and were included in the meta-analysis. Sixteen studies were analyzed only qualitatively since they used different techniques from the ones eligible for inclusion in meta-analysis or had insufficient information regarding one or 2 of the outcomes or used different co-culture systems.27-31,33,34,36,39-46
Figure 1.
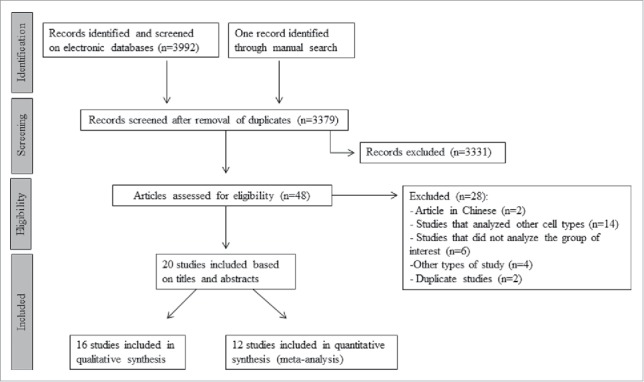
Flowchart illustrating the search strategy used to identify studies for inclusion in the systematic review and meta-analysis.
We assessed the quality of each individual study included in the present meta-analyses using the Grading of Recommendation Assessment, Development and Evaluation (GRADE).47 For each outcome, quality of evidence was classified as high, moderate, low or very low. Studies included in our meta-analyses are not-blinded. Therefore, using the GRADE recommendations, the evidence was classified as having low to very low quality for both outcomes.
Studies included in qualitative analysis
Sixteen studies27-31,33,34,36,39-46 were only qualitatively described. Table 1 summarizes the results obtained by those studies regarding islet viability and/or insulin secretion. For all studies included in Table 1, the test condition (MSCs plus islets) was compared with islet cultured alone (control group). Most of those studies (56.3%) analyzed murine islets co-cultured with murine bone marrow (mBM) derived MSCs. Other studies analyzed murine islets co-cultured with human umbilical cord blood (hUCB), murine kidney (mKidney), or murine adipose-derived stem/stromal cells (mASCs). Only one study evaluated pig islets co-cultured with human ASCs (hASCs). Three studies used an indirect co-culture system (where the islets were separated from the MSC monolayer by a semi-permeable membrane in a transwell system, allowing the passage of soluble factors, Fig. 2C); 5 other studies used a direct co-culture system (islets were cultured in direct contact with MSCs in monolayer or in suspension; Fig. 2A and B); while 3 studies evaluated both direct and indirect co-culture systems (Table 1). Five studies used different types of co-culture systems. Lin et al.41 used a co-culture microfluidic chip where mBM-MSCs and islets were introduced respectively into 2 microchambers which could be connected by a traffic tunnel. Three studies encapsulated murine islets together with syngeneic MSCs,39,43,44 and one study mixed murine islet single cells or intact islets with mASCs in concave microwells to have 3D co-cultured islet spheroids.45
Table 1.
Summary of the results for the studies analyzed only qualitatively.
| Results (Islets + MSCs vs. Islet alone) |
||||
|---|---|---|---|---|
| Author / year | Islet / MSC origin | Type of co-culture system | Viability | Insulin secretion |
| Park et al.27 2010 | Murine / hUCB | Indirect | ↑ viability in 7 d (AO/PI); ↓ DNA fragmentation (ELISA) in 2 d | ↑ GSIS on the 2nd day (ELISA) |
| Karaoz et al.29 2010 | Murine / mBM | Indirect | ↓ apoptosis on the 14th day (FACS - Annexin V/PI) | No difference in GSIS on the 14th day (ELISA) |
| Jung et al.30 2011 | Murine / mBM | Indirect | ↑viability on the 7th day (FDA/PI) | No difference in GSIS on the 14th and 30th days; small, although not significant, increase in intracellular insulin content on the 14th day (ELISA) |
| Direct (suspension) |
↑ viability at 7 d (FDA/PI) when compared with both control and indirect contact groups | ↑ GSIS on the 14th and 30th days; ↑ insulin content in 14th day when compared with both control and indirect contact groups | ||
| Karaoz et al.42 2011 | Murine / mBM | Direct (monolayer) | — | ↑ basal insulin (ELISA) after 16 days |
| Chen et al.28 2013 | Murine / mBM | Direct (suspension) |
↑ viability during 30 d (AO/PI) | No differences on the 1st and 3th day; ↑ GSIS after 7 d (immunoradiometric assay) |
| Rackham et al.51 2013 | Murine / mKidney | Indirect | — | No differences in GSIS and insulin content in 3 d (radioimmunoassay) |
| Direct (suspension) |
— | ↑ GSIS (3rd day), but it was not significantly different from the control group | ||
| Direct (monolayer) | — | ↑ insulin content on the 3rd day compared with control, and ↑ GSIS compared with both control and direct contact groups | ||
| Rackham et al.46 2014 | Murine / mASC | Direct (monolayer) | — | ↑ GSIS on the 3rd day (radioimmunoassay); no differences in insulin content for the same period |
| Scuteri et al.36 2014 | Murine / mBM | Indirect | — | No differences on GSIS (ELISA) |
| Direct (suspension) |
— | ↑ GSIS after 14 and 21 d, but not on the 28th day | ||
| Yamada et al.33 2014 | Pig / hASC | Indirect | — | ↑ basal insulin on the 2nd day (ELISA) |
| Yoshimatsu et al.40 2015 | Murine / mBM | Direct (suspension) |
— | ↑ GSIS (4rd day), but it was not significantly different from the control group |
| Rackham et al.34 2016 | Murine / mASC | Direct (monolayer) | — | ↑ GSIS on the 3rd day (radioimmunoassay) |
| Other types of co-culture systems | ||||
| Lin et al.41 2009 | Murine / mBM | Microfluidic chip with microchambers | — | ↑ GSIS from 3 to 21 d (ELISA) |
| Davis et al.43 2012 | Murine / mBM | Encapsulation with silk hydrogel | — | ↑ ISI, although with only a marginal significance (P = 0.06) (ELISA) |
| Kerby et al.44 2013 | Murine / mKidney | Encapsulation with alginate | — | ↑ GSIS and insulin content on the 3rd day (radioimmunoassay) |
| Yoshimatsu et al.39 2013* | Murine / mBM | Encapsulation with PVA | No differences on viability on the 3rd day (Syto-Green/EB) | No differences on ISI in 4 d (ELISA) |
| Jun et al.45 2014 | Murine / mASC | Co-culture in microchip microwells | No differences on the 3rd day, but ↑ viability on the 7th and 14th days compared with intact islets and dispersed islets (calcein AM/EH 1) | No differences on 3rd day, but ↑ ISI on the 7rd and 14rd days compared with both intact and dispersed islets (ELISA) |
MSC, mesenchymal stem/stromal cells; h, human; m, murine; BM, bone marrow; UCB, umbilical cord blood; ASC, adipose-derived stem/stromal cells; GSIS, glucose stimulated insulin secretion; ISI, insulin stimulation index; AO, acridine orange; PI, propidium iodide; FDA, fluorescein diacetate; EB, ethidium bromide; EH 1, ethidium homodimer 1. * This study also evaluated direct co-culture system, which results were included in the viability and ISI meta-analysis.
Figure 2.
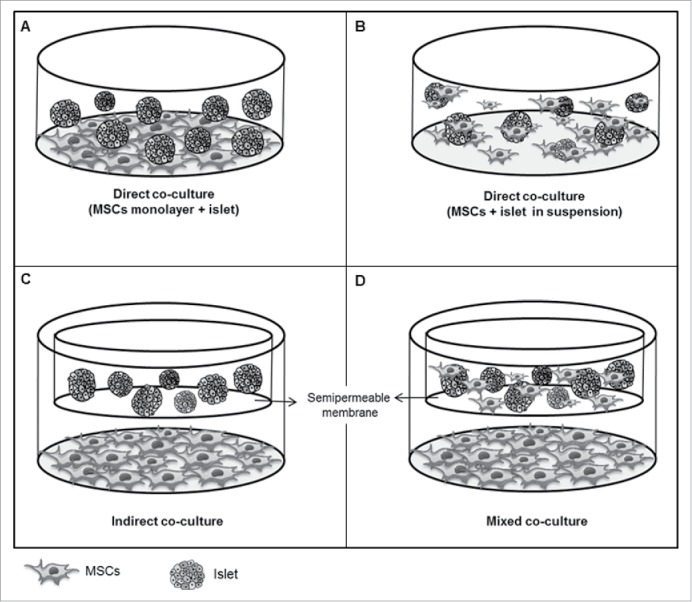
Schematic representation of different culture systems of pancreatic islets with MSCs. (A) Direct contact system with MSCs in monolayer. In this system, MSCs are plated to the culture dish covering the bottom of it; then, hours late, islets are plated and enter in direct contact with MSCs. (B) Direct contact system (MSCs and islets in suspension), where islets and MSCs are added together in the culture medium at the same time. Late, some MSCs can coat the islets. (C) Indirect contact system, where islets are separated from the MSC monolayer by a semi-permeable membrane in a transwell system, allowing the passage of soluble factors. (D) Mixed contact system, where MSCs were both in direct and indirect contact with islets. Of note, MSCs are in direct contact with islets in a transwell and also in indirect contact through adherence of MSCs to the bottom of the dish.
Most studies reported increased viability in murine islets co-cultured with MSCs (7 to 30 d) as compared with control condition, both for indirect,27,30 or direct28,30 co-culture systems (Table 1). Karaoz et al.29 showed decreased apoptosis after 14 d of co-culture of murine islets with mBM-derived MSCs. In the same way, Park et al.27 reported a decrease in DNA fragmentation after 2 d of co-culture of murine islets with hUCB. In the other hand, 2 studies reported no differences in viability between murine islets co-cultured with syngeneic MSCs or islets cultured alone for 3 d.39,45 However, one of these studies was able to show an increase in viability of islet spheroids exposed to mASCs compared with intact and single islet spheroids after 7 and 14 d in concave microwells.45
A direct co-culture of islet with MSCs (in monolayer or in suspension) also seems to increase basal insulin and glucose-stimulated insulin secretion (GSIS)28,30,31,34,36,40,42,46 (Table 1). The benefit of an indirect co-culture system on islet function is still controversy, with some studies reporting an increase in basal insulin or GSIS,27,33 while other studies29-31,36 were not able to show any differences in these outcomes when compared with islets cultured alone. Lin et al.41 using a co-culture microfluidic chip, reported that mBM-derived MSCs had the ability to migrate to the microchamber containing murine islets and provide an improvement of GSIS during 3 to 21 d of culture. Jun et al.45 after mixing murine islets with mASCs in concave microwells, creating 3D-islet spheroids, suggested an increase in GSIS and ISI (7th and 14th d) when compared with intact islets or single islets spheroids. Kerby et al.44 observed that alginate-encapsulated islets with mKidney-derived MSCs had increased GSIS and intracellular insulin content after 3 d of culture when compared with islets encapsulated alone. However, 2 other studies that encapsulated islets with mBM-MSCs were not able to show any significant improvement in GSIS or ISI.39,43
Studies included in meta-analyses
Supplementary Table 1 summarizes the main characteristics of the 12 studies selected for inclusion in the meta-analysis. Among them, 4 studies analyzed the viability outcome,29,33,35,36 6 analyzed the ISI outcome26-28,30,37,38 and 2 analyzed both outcomes.39,40
Table 2 shows the pooled weighted mean differences (WMD) comparing the effect of co-culture of islets with MSCs and islet cultured alone for both viability and ISI outcomes. Fig. 3 illustrates the pooled WMD for viability after stratification for co-culture systems. Overall, the mean of viability was higher in islets co-cultured with MSCs as compared with islet alone [WMD = 18.08 (95% CI: 12.59 – 23.57)]. This improvement in islet viability showed to be higher in islets co-cultured in an indirect or mixed contact with MSCs than in islets having a direct contact with MSCs (P <0.001 for comparisons among subgroups) (Table 2). Mixed co-culture method means that both direct and indirect systems coexist, to know, the MSCs are in direct contact with pancreatic islets in a transwell, and also in indirect contact through adherence of MSCs to the bottom of the dish (monolayer) (Fig. 2D).
Table 2.
Pooled measures for the effect of the co-culture of pancreatic islets and MSCs on viability and ISI WMD.
| Viability |
ISI |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Number of groups analyzed | I2 (%) | Pooled WMD (95% CI) | P-value* | Number of groups analyzed | I2 (%) | Pooled WMD (95% CI) | P-value* |
| Overall | 18 | 97.8 | 18.08 (12.59 – 23.57) | — | 15 | 69.3 | 0.83 (0.54 – 1.13) | — |
| Type of co-culture | < 0.001 | 0.224 | ||||||
| Indirect contact | 7 | 97.6 | 23.84 (15.99 – 31.69) | a | 4 | 75.9 | 1.10 (0.52 - 1.68) | |
| Direct contact | 7 | 52.5 | 5.93 (3.26 – 8.60) | b | 11 | 60.9 | 0.68 (0.35 – 1.02) | |
| Mixed contact | 4 | 98.2 | 28.84 (15.70 – 41.98) | a | — | — | — | |
| Islet origin | 0.907 | 0.493 | ||||||
| Murine | 17 | 97.9 | 18.05 (12.37 – 23.74) | 12 | 69.7 | 0.78 (0.44 – 1.12) | ||
| Pig | 1 | — | 18.60 (11.39 – 25.81) | — | — | — | ||
| Human | — | — | — | 3 | 73.0 | 1.11 (0.24 - 1.97) | ||
| MSCs origin | 0.332 | 0.114 | ||||||
| Murine | 16 | 97.9 | 18.71 (12.87 – 24.56) | 11 | 61.0 | 0.66 (0.35 – 0.98) | ||
| Human | 2 | 85.6 | 12.65 (1.87 – 23.43) | 4 | 72.9 | 1.23 (0.60 – 1.86) | ||
| MSCs tissue origin | 0.332 | < 0.001 | ||||||
| BM | 16 | 97.9 | 18.71 (12.87 – 24.56) | 13 | 53.7 | 0.65 (0.38 – 0.91) | ||
| UCB | — | — | — | 2 | 0.0 | 1.56 (1.17 – 1.96) | ||
| Adipose | 2 | 85.6 | 12.65 (1.87 – 23.43) | — | — | — | ||
MSC, mesenchymal stromal cells; ISI, insulin stimulation index; WMD, unstandardized weighted mean difference.
P-value for subgroup (univariate) analysis. Letters a and b refer to pairwise comparisons between co-culture contact system. Groups with different letters are significantly different (P < 0.05, Wald test).
Figure 3.
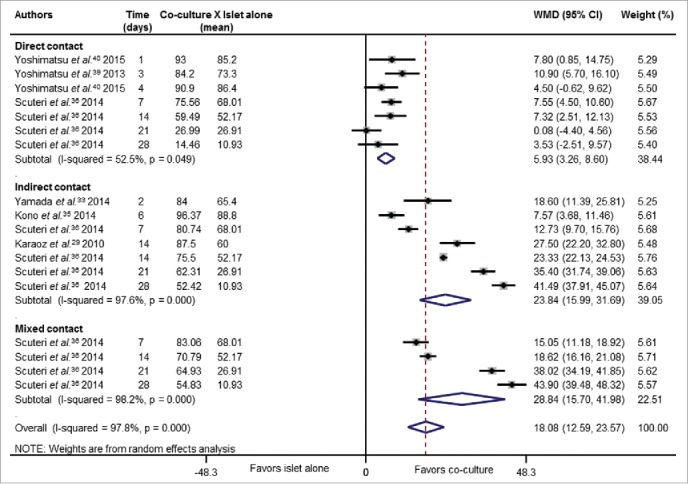
Forest plot showing individual and pooled WMD (95% CI) for the effects of co-culture of islets and MSCs on islet viability. Results were analyzed accordingly to different co-culture systems (indirect, direct or mixed contact). Areas of the squares reflect the weight of each individual study and the diamonds illustrate random effect WMDs (95% CI) estimated from the studies. Co-culture groups were compared with islet cultured alone. Some studies were included more than once in the analysis since they analyzed different periods of culture.
Fig. 4 and Table 2 show the pooled WMD for the ISI outcome after stratification for tissue origin of MSCs. Accordingly to the viability results, the mean of ISI was significantly higher in islets co-cultured with MSCs than in islets cultured alone [WMD = 0.83 (95% CI: 0.54 - 1.13)]. Interestingly, the mean of ISI was higher in islets co-cultured with UCB-derived MSCs [WMD = 1.56 (95% CI 1.17 - 1.96)] than in islets co-cultured with BM-derived MSCs [WMD = 0.65 (95% CI 0.38 - 0.91)] (P <0.001).
Figure 4.
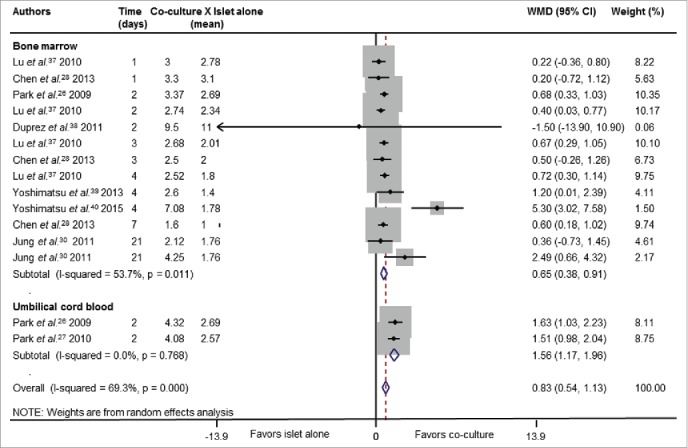
Forest plot showing individual and pooled WMD (95% CI) for the effects of co-culture of islets and MSCs on ISI. Results are shown after stratification by tissue source of MSCs. Areas of the squares reflect the weight of each individual study and the diamonds illustrate random effect WMDs (95% CI) estimated from the studies. Co-culture groups were compared with islet cultured alone. Some studies were included more than once in the analysis since they analyzed different periods of culture.
As shown in Table 2, there were significant heterogeneities (I2 > 50%) among studies in almost all sub-group comparisons of viability and ISI and also in the overall analyses (viability: I2 = 97.8%, ISI: I2 = 69.3%). To investigate these findings, bivariate meta-regression analyses were performed as described in the Statistical analysis for meta-analysis section. In these analyses, interactions between one qualitative characteristic at a time (type of co-culture system, islet origin, MSCs species origin, and MSCs tissue origin) and co-culture time were investigated for each outcome. Fig. 5A shows that there is an improvement in viability mean differences overtime for islets co-cultured with MSCs in indirect or mixed contact systems (both P <0.001), and a worsening in viability for islets co-cultured in direct contact systems (P = 0.005). Fig. 5B demonstrates that islets co-cultured with MSCs show higher mean difference of ISI overtime in the direct contact system than islet cultured alone (P = 0.02), while no significant difference overtime was observed for islets co-cultured in indirect contact systems compared with islet cultured alone (P = 0.29). Visual inspection of funnel plots did not indicate asymmetry suggestive of lack of small-study bias for both analyzed outcomes (data not shown). Moreover, no significant publication bias was detected for viability (P = 0.62) and ISI (P = 0.52) outcomes by the Begg and Egger test, suggesting that our data are statistically robust.
Figure 5.
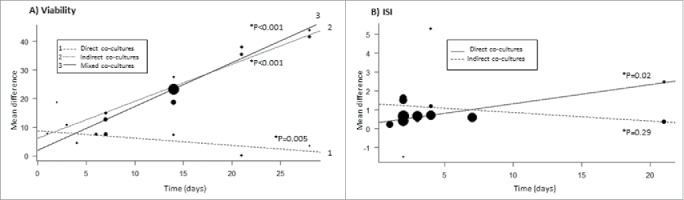
Bivariate metaregression models with time of culture (in days) and type of co-culture system as predictors for viability (A) and ISI (B) outcomes. The area of the circles reflects the weight of each individual study. (A) Effect size of indirect, direct and mixed co-culture systems on viability overtime. *P-values are related to comparison between islets co-cultured with MSCs vs. islet cultured alone. The effect observed for mixed co-culture system was similar to that obtained for the indirect co-culture systems (P = 0.42), and both effects were different to that obtained for the direct contact system (both P < 0.001). (B) Effect size of indirect and direct co-culture systems on the ISI outcome overtime.*P-values are related to the comparison between co-culture condition vs. islet alone condition. The effect observed for the direct and indirect co-culture systems were different (P = 0.02).
Discussion
Successful islet transplantation is not only dependent on the number of transplanted islets, but also on their quality.11,48 Thus, a strategy for preserving isolated islets without reducing their viability or function during culture period could be invaluable.30 In this context, several studies have suggested that co-culture of islets with MSCs can enhance β cell viability and function and, consequently, islet graft survival.26-28,30,31,33,35-37,46 Many of the protective effects of MSCs could be mediated by secretion of paracrine factors.26-28,33,37,49 However, some studies suggest that a direct physical contact between islets and MSCs is critical to improve islet survival, structural integrity and insulin function.30,31,34,36,44,45 At the present time, it is still inconclusive if paracrine factors are enough to improve islet quality or if a physical contact with islets is needed. Therefore, taking into account that meta-analysis has been considered a powerful tool for pooling the results from different studies,50 we performed a systematic review and/or meta-analysis of 20 published articles that compared the effect of direct or indirect co-culture of islets with MSCs on islet quality outcomes.
Our meta-analysis results suggest that viability is higher in islets co-cultured with MSCs than islets cultured alone, which is independent of the type of contact system used. This data are in agreement with results reported by most studies analyzed qualitatively by us (Table 1).27-30,43,45 Interestingly, this improvement in viability seems to be significantly higher overtime for those islets co-cultured in indirect or mixed contact systems compared with islets co-cultured in a direct contact with MSCs (Fig. 5A). The underlying mechanisms modulating increased islet viability and cell membrane integrity might be attributed to the paracrine mediators secreted by MSCs, including vascular endothelial growth factor (VEGF), insulin-like growth factor 1, transforming growth factor β 1, hepatocyte growth factor, hemeoxygenase-1, interleukin 6, tissue inhibitor of metalloproteinase (TIMP)-1, and indoleamine 2, 3-dioxygenase, among others.26,27,29,33,35,49
Park et al.27 reported that murine islets co-cultured with hUCB-MSCs showed an upregulation of anti-apoptotic signaling molecules [X-linked inhibitor of apoptosis protein, β cell CLL/lymphoma-2, BCL−XL, and heat shock protein 32], probably resulting from Akt pathway activation, which is known to play a crucial role in survival of β cells. At the mRNA level, they observed increased expressions of angiogenesis/revascularization-related genes [VEGF receptor 2, induced by VEGF, and TEK tyrosine kinase endothelial (Tek/Tie-2)] in islets from the co-cultured condition. Yamada et al.33 reinforced the importance of VEGF for islet survival in vitro via inhibition of VEGF function using bevacizumab. They showed that islet viability was significantly lower in the co-culture group (porcine islets + hASCs) treated with bevacizumab than in non-treated co-culture condition. They concluded that VEGF might correlate with vasculogenesis and angiogenesis as well as anti-apoptotic effects. In the study by Kono et al.35 analysis of hASC-derived factors revealed VEGF and TIMP-1 to be highly abundant proteins secreted by hASCs in the presence of stressed murine islets in an indirect co-culture system. TIMP-1 is a member of the matrix metalloproteinase inhibitor family and is known to regulate several biologic processes, such as cell growth, migration, and apoptosis. Accordingly, TIMP-1 blockade was able to abrogate, in vitro, pro-survival effects of hASCs, suggesting an important role for TIMP-1 in β cell survival under pro-inflammatory cytokine stress.35 TIMP-1 and VEGF were also increased in murine islet co-cultured for 4 weeks with mBM-MSCs, both for indirect and direct contact systems compared with islet cultured alone condition, which was associated to a decrease in chemokine (C-C motif) ligand 2 (CCL2 or MCP1) and tumor necrosis factor levels in the culture medium.30
Our meta-analysis results also indicate that islet co-cultured with MSCs show an improvement in insulin secretion (ISI) compared with islet cultured alone, independently of the type of contact system used. However, when taking into account the duration of co-culture, a direct physical contact between islets and MSCs seems to be more effective in improving islet secretory function in long-term culture than in an indirect contact system (Fig. 5B). This last result should be interpreted with caution due to the small number of studies that analyzed islets kept more than 7 d in culture. Only few studies have directly compared different co-culture conditions. 30,31,36 However, these studies indicate that a direct islet-MSC contact is more efficient in improving insulin secretion overtime than the indirect contact between cells, which is in agreement with our meta-analysis data.
Scuteri et al.36 compared direct, indirect and mixed co-cultures of murine islets with mBM-MSCs during 1 to 4 weeks of follow-up. Although the direct contact did not influence islet's survival, it was able to trigger pancreatic and duodenal homeobox (Pdx)-1 expression in MSCs, which is a pivotal gene regulating insulin production. Pdx-1 triggered the differentiation of MSCs into insulin-releasing cells and, consequently, increased GSIS in the culture medium after 2–3 weeks of culture. On the other hand, in indirect co-cultures, only an increase in islet viability was observed, probably mediated by trophic factors released by MSCs. Interestingly, the mixed contact system was associated with an increase in both viability and insulin secretion, uniting the distinct mechanisms of action in a single paradigm.36
Likewise, Jung et al.30 showed that physical contact between mBM-MSCs and murine islets was more effective in sustaining islet secretory function in long-term culture (2–3 weeks) than the indirect contact or islet monoculture. They concluded that in the direct contact group, MSCs aggregated around the islets and formed a capsule-like structure that probably preserved islet morphology, providing a microenvironment suitable for the repair of islet injury and supporting islet function.30 Moreover, Rackham et al.31 demonstrated that the indirect contact of murine islets with mKidney-MSCs did not improve islet function, probably because soluble factors alone cannot account for the beneficial impact of MSCs in insulin secretion. However, the use of a direct contact configuration (with MSC monolayer) improved GSIS in vitro due to a dynamic cross-talk between those cells, which correlated with superior islet graft in vivo. The results of other studies analyzed only qualitatively by us also indicate that the direct configuration is efficient for improving GSIS,34,42,46 while the indirect contact system is not.29 Unfortunately, these last studies did not compare different co-culture systems.
Of note, some studies evaluated the effects of islet co-culture with MSCs in different in vitro stress conditions, further corroborating that MSCs protect islets during culture period. Lu et al.37 reported that MSCs protected islets from hypoxia/reoxygenation-induced injury by decreasing apoptosis, increasing hypoxia-inducible factor-1α, cyclooxygenase-2 and heme oxygenase-1 expressions, and improving GSIS. Other studies also showed that MSC co-culture improved islet viability and/or function after exposure to pro-inflammatory cytokines35,51 or streptozotocin.29
Although the evaluation of the in vivo effects of co-culture of islets with MSCs was not the aim of this systematic review and meta-analysis, some of the included studies also reported data regarding transplantation in diabetic mice of co-cultured islets with MSCs compared with islets cultured alone. Two studies showed that the transplantation of islets pre-cultured with MSCs in a direct contact system (monolayer) was associated with a significant improvement in glycemic control of mice compared with transplantation of islets cultured alone.31,46 In addition, islets from the co-culture condition had a 60% increase on the capacity to reverse hyperglycemia in a period of 30 d after transplantation.31,46 These in vivo functional data are in accordance with the results of our meta-analysis.
Taking into account both islet viability and function data, we therefore suggest that for short time cultures (until 2–3 d, as commonly used in the islet transplantation setting), all type of contact systems may be used for the improvement of these outcomes. However, for longer culture periods, direct physical contact seems to be more effective than indirect contact in sustaining islet secretory function. Possibly, the mixed contact system might have an advantage in relation to the other systems since it unites positive effects of the trophic factors secreted by MSCs as well as the differentiation of MSCs into insulin-secreting cells, as proposed by Scuteri et al.36
The present systematic review and meta-analysis has some limitations. First, heterogeneity is potentially a significant problem when interpreting the results of any meta-analysis, and the present meta-analysis showed significant inter-study heterogeneities for the 2 outcomes analyzed. The heterogeneity can be explained by differences in the source of isolated pancreatic islets (human, murine or pig) and MSCs (human or murine) or tissue origin of MSCs (adipose tissue, BM, UCB) as well as different experimental protocols regarding co-culture duration or differential experience of the research groups in a given method, MSCs passage number, details of the contact system used, and techniques used for evaluation of viability or insulin secretion. Second, the ratio of MSCs to islets may have an important effect on islet outcomes37 since it could be related to the amount of MSC-secreted soluble mediators. However, the MSC/islet ratio was variable among the studies, and some studies did not provide this information. Therefore, this ratio could not be included as a qualitative predictor in meta-regression or subgroup analyses. Third, some studies did not describe important information regarding experimental design, which difficult the interpretation of the results. Fourth, using GRADE recommendations, most studies were considered as having low to very low quality since they were not clinical blinded studies. Fifth, due to differences in the experimental methods some studies could be analyzed only qualitatively; therefore, only a few studies were available for inclusion in the meta-analysis. Sixth, meta-analysis is notoriously prone to publication bias, and although we have attempted to trace unpublished observations and the statistical tests did not indicate risk of publication bias, we cannot be sure that small negative studies were overlooked. However, we believe that this systematic review and meta-analysis is valid to synthetize the information regarding the effect of co-culture of MSCs with islets.
In conclusion, this study has shown that co-culture of pancreatic islets with MSCs has the potential for protecting islets from injury after isolation and during culture period independent of the type of contact system used. Therefore, this might be a valuable method for β cell preservation before transplantation, decreasing the loss of islets, which currently limits the application of allogeneic islet transplantation as a more widespread therapy for T1DM.
Materials and Methods
Search strategy and eligibility criteria
A systematic review was designed and described in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.52 To identify all studies that reported co-culture of pancreatic islets with MSCs, we performed an electronic literature search in PubMed and Embase repositories, without data restriction. The following medical subject headings (MeSH) were used for this search: (“mesenchymal stem cell” OR “mesenchymal stromal cell” OR “adult stem cell” OR “multipotent stem cell” OR “stem cell” OR “mesenchymal stem cell transplantation” OR “stem cell research”) AND (“pancreas islet” OR “islets of Langerhans” OR “pancreas islet transplantation” OR “islet transplantation”). The search was restricted to English and Spanish language papers and was completed on May 5, 2016. All articles retrieved were also searched manually to identify any other relevant citations.
Eligibility evaluation was done by title and abstracts review and when abstracts did not provide sufficient information, the full text of the paper was retrieved for analysis. This was performed independently in a standardized manner by 2 investigators (K.R. and A.P.B.). Discrepancies were resolved by discussion between them and, when needed, a third reviewer (D.C.) was consulted. Where abstracts did not provide sufficient information about the inclusion criteria, a further full text analysis was done. Studies were considered eligible for inclusion if they matched the following criteria: 1) the study should have compared outcomes of pancreatic islets and MSCs co-culture (case group) with results of islets cultured alone (control group); 2) it should have analyzed markers of islet viability and/or islet function in vitro, and 3) it should be an original article. Whether data were duplicated or had been published more than once, the most complete study was chosen. For articles with missing information, the authors were contacted for further information, but the majority of them did not answer. Articles that did not fulfill the eligibility criteria described above were excluded from the study.
Data extraction and quality control assessment
Data were independently extracted by 2 investigators (B.M.S. and A.P.B.) using a standardized abstraction form, and consensus was sought in all extracted items. When consensus could not be achieved, differences in data extraction were resolved by a third reviewer (D.C.) and by reading the original publication. Information extracted from each study were as follow: name of first author, publication year, islet origin (human / murine / pig), MSCs origin (human / murine), tissue from which MSCs were extracted (e.g. BM / UCB / adipose tissue / kidney), method of co-culture (indirect / direct / mixed; Fig. 2), culture duration, number of replicates analyzed in each group and results of islet viability and function (in mean ± SD, SE or through estimation from graphics that had been found in the articles).
For the viability outcome, data obtained from techniques that used comparable staining dyes for viable/dead cells [fluorescein diacetate (FDA) / propidium iodide (PI); acridine orange (AO)/PI; Syto-Green/ethidium bromide (EB); calcein-AM; ethidium homodimer-1 (EH 1)/calcein-AM or trypan blue] were compiled in the quantitative analysis. Studies that used DNA-fragmentation-based ELISA kits or FACS-analysis for determination of apoptosis were included only in the qualitative analysis. For the islet function outcome, all studies that calculated ISI – rate of high to low glucose stimulated-insulin secretion) were included in the quantitative analysis. Studies that only measured basal or GSIS or intracellular insulin content were just qualitatively analyzed.
Two investigators (B.M.S and A.P.B.) independently assessed the quality of each eligible study using GRADE recommendations.47 GRADE categorizes quality of evidence into 4 categories: high, moderate, low or very low. The quality evaluation includes factors such as the study design (risk of bias), imprecision, inconsistency, indirectness, and publication bias. Risk of publication bias was assessed using funnel plot graphics, analyzed both visually and with the Begg and Egger test.53 The significance of the intercept was determined by the t test, as proposed by Egger, with P <0.10 considered indicative of statistically significant publication bias.
Statistical analysis for meta-analysis
Outcomes of interest included in the meta-analyses were islet viability and ISI. For both outcomes, pooled results are shown as unstandardized WMD between islets cultured alone and the co-culture condition (MSCs plus islets). Heterogeneity among studies was tested using the Cochran's Q test and the I2 (inconsistency) statistic.54,55 Inter-study heterogeneity was considered statistically significant at P <0.10 (Q test) and I2 >50%. Due to different experimental conditions across studies, significant heterogeneities are expected. Therefore, random effects model, with DerSimonian and Laird estimator and the inverse variance method, was used to calculate WMD, 95% CI and P values.54,55
To further explore the expected heterogeneities, bivariate meta-regression or subgroup meta-analyses were carried-out to assess possible associations between different variables (qualitative and quantitative predictors) and outcomes. Qualitative predictors used in subgroup (univariate) analysis were type of co-culture system (indirect, direct or mixed contact), islet origin (murine, human or pig), MSCs species origin (murine or human), and MSCs tissue origin (BM, UCB or adipose tissue), while the quantitative predictor was time (duration of culture in days). The significance of each individual predictor was tested using the likelihood ratio test.56 In addition, taking into account that the type of co-culture system predictor had more than 2 categories, Wald test with Bonferroni adjustment was used for pairwise comparisons.56 To further explore the effect of possible interactions between time and the 4 qualitative predictors on the outcomes, bivariate meta-regression analyses using mixed-effect models were also performed.
Statistical analyses were performed using Stata 11.0 software (StataCorp, College Station, TX, USA) and the R software (Meta and Metafor packages; R Core Team, Vienna, Austria).
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was partially supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundo de Incentivo à Pesquisa e Eventos (FIPE) at the Hospital de Clínicas de Porto Alegre and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- [1].American Diabetes Association Classification and diagnosis of diabetes. Diabetes care 2016; 38:S8-S16; http://dx.doi.org/ 10.2337/dc15-S005 [DOI] [PubMed] [Google Scholar]
- [2].The Diabetes Control and Complications Trial Research Group . Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997; 46:271-286; PMID: 9000705; http://dx.doi.org/ 10.2337/diab.46.2.271 [DOI] [PubMed] [Google Scholar]
- [3].The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977-986; PMID: 8366922; http://dx.doi.org/ 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- [4].Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000; 343:230-238; PMID: 10911004; http://dx.doi.org/ 10.1056/NEJM200007273430401 [DOI] [PubMed] [Google Scholar]
- [5].Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes 2005; 54:2060-2069; PMID: 15983207; http://dx.doi.org/ 10.2337/diabetes.54.7.2060 [DOI] [PubMed] [Google Scholar]
- [6].Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, et al.. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes care 2012; 35:1436-1445; PMID: 22723582; http://dx.doi.org/ 10.2337/dc12-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leitao CB, Tharavanij T, Cure P, Pileggi A, Baidal DA, Ricordi C, Alejandro R. Restoration of hypoglycemia awareness after islet transplantation. Diabetes care 2008; 31:2113-2115; PMID: 18697903; http://dx.doi.org/ 10.2337/dc08-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant 2008; 8:1990-1997; PMID: 18828765; http://dx.doi.org/ 10.1111/j.1600-6143.2008.02353.x [DOI] [PubMed] [Google Scholar]
- [9].Ricordi C. Islet transplantation: a brave new world. Diabetes. 2003; 52:1595-1603; PMID: 12829621; http://dx.doi.org/ 10.2337/diabetes.52.7.1595 [DOI] [PubMed] [Google Scholar]
- [10].McCall M, James Shapiro AM. Update on islet transplantation. Spring Harb Perspect Med 2012; 2:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ichii H, Wang X, Messinger S, Alvarez A, Fraker C, Khan A, Kuroda Y, Inverardi L, Goss JA, Alejandro R. et al.. Improved human islet isolation using nicotinamide. Am J Transplant 2006; 6:2060-2068. [DOI] [PubMed] [Google Scholar]
- [12].Marzorati S, Antonioli B, Nano R, Maffi P, Piemonti L, Giliola C, Secchi A, Lakey JR, Bertuzzi F. Culture medium modulates proinflammatory conditions of human pancreatic islets before transplantation. Am J Transplant 2006; 6:2791-2795; PMID: 16939517; http://dx.doi.org/ 10.1111/j.1600-6143.2006.01512.x [DOI] [PubMed] [Google Scholar]
- [13].Kin T, Senior P, O'Gorman D, Richer B, Salam A, Shapiro AM. Risk factors for islet loss during culture prior to transplantation. Transpl Int 2008; 21:1029-1035. PMID: 18564983 [DOI] [PubMed] [Google Scholar]
- [14].Ricordi C, Fraker C, Szust J, Al-Abdullah I, Poggioli R, Kirlew T, Khan A, Alejandro R. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation 2003; 75:1524-1527; PMID: 12792508; http://dx.doi.org/ 10.1097/01.TP.0000058813.95063.7A [DOI] [PubMed] [Google Scholar]
- [15].Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas 2000; 20:270-276; PMID: 10766453; http://dx.doi.org/ 10.1097/00006676-200004000-00008 [DOI] [PubMed] [Google Scholar]
- [16].Goto M, Yoshikawa Y, Matsuo K, Shirasu A, Ogawa N, Takahashi H, Saitoh Y, Fujimori K, Kurokawa Y, Tamai M, et al.. Optimization of a prominent oxygen-permeable device for pancreatic islets. Transplant Proc 2008; 40:411-412; PMID: 18374084; http://dx.doi.org/ 10.1016/j.transproceed.2008.01.056 [DOI] [PubMed] [Google Scholar]
- [17].Ilieva A, Yuan S, Wang RN, Agapitos D, Hill DJ, Rosenberg L. Pancreatic islet cell survival following islet isolation: the role of cellular interactions in the pancreas. J Endocrinol 1999; 161;357-364; PMID: 10333538; http://dx.doi.org/ 10.1677/joe.0.1610357 [DOI] [PubMed] [Google Scholar]
- [18].Ling Z, Hannaert JC, Pipeleers D. Effect of nutrients, hormones and serum on survival of rat islet beta cells in culture. Diabetologia 1994; 37:15-21; PMID: 7512059; http://dx.doi.org/ 10.1007/BF00428772 [DOI] [PubMed] [Google Scholar]
- [19].Miki A, Narushima M, Okitsu T, Takeno Y, Soto-Gutierrez A, Rivas-Carrillo JD, Navarro-Alvarez N, Chen Y, Tanaka K, Noguchi H, et al.. Maintenance of mouse, rat, and pig pancreatic islet functions by coculture with human islet-derived fibroblasts. Cell transplantation 2006; 15:325-334; PMID: 16898226; http://dx.doi.org/ 10.3727/000000006783981882 [DOI] [PubMed] [Google Scholar]
- [20].Yang Z, Chen M, Carter JD, Ellett JD, Smith KM, Nadler JL. Inflammation blockade improves pancreatic islet function. Transplant Proc 2004; 36:2864-2865; PMID: 15621169; http://dx.doi.org/ 10.1016/j.transproceed.2004.09.083 [DOI] [PubMed] [Google Scholar]
- [21].Sharma A, Sorenby A, Wernerson A, Efendic S, Kumagai-Braesch M, Tibell A. Exendin-4 treatment improves metabolic control after rat islet transplantation to athymic mice with streptozotocin-induced diabetes. Diabetologia 2006; 49:1247-1253; PMID: 16609877; http://dx.doi.org/ 10.1007/s00125-006-0251-2 [DOI] [PubMed] [Google Scholar]
- [22].Chao KC, Chao KF, Chen CF, Liu SH. A novel human stem cell coculture system that maintains the survival and function of culture islet-like cell clusters, Cell transplantation 2008; 17:657-664. [DOI] [PubMed] [Google Scholar]
- [23].Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A, Feili-Hariri M. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun 2009; 32:116-124; PMID: 19217258; http://dx.doi.org/ 10.1016/j.jaut.2009.01.003 [DOI] [PubMed] [Google Scholar]
- [24].Gatto C, Callegari M, Folin M, Conconi M, Paolin A, Di Falco G, Bredariol S, Spinazzi R, Parnigotto PP, Nussdorfer GG. Effects of cryopreservation and coculture with pancreatic ductal epithelial cells on insulin secretion from human pancreatic islets. Int J Mol Med 2003; 12:851-854. PMID: 14612956 [PubMed] [Google Scholar]
- [25].Hematti P, Kim J, Stein AP, Kaufman D. Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplantation reviews (Orlando) 2013; 27:21-29; http://dx.doi.org/ 10.1016/j.trre.2012.11.003 [DOI] [PubMed] [Google Scholar]
- [26].Park KS, Kim YS, Kim JH, Choi BK, Kim SH, Oh SH, Ahn YR, Lee MS, Lee MK, Park JB, et al.. Influence of human allogenic bone marrow and cord blood-derived mesenchymal stem cell secreting trophic factors on ATP (adenosine-5′-triphosphate)/ADP (adenosine-5′-diphosphate) ratio and insulin secretory function of isolated human islets from cadaveric donor. Transplant Proc 2009; 41:3813-3818; PMID: 19917393; http://dx.doi.org/ 10.1016/j.transproceed.2009.06.193 [DOI] [PubMed] [Google Scholar]
- [27].Park KS, Kim YS, Kim JH, Choi B, Kim SH, Tan AHK, Lee MS, Lee MK, Kwon CH, Joh JW, et al. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation 2010; 89:509-517; PMID: 20125064; http://dx.doi.org/21364643 10.1097/TP.0b013e3181c7dc99. [DOI] [PubMed] [Google Scholar]
- [28].Chen J, Ye YF, Liao LM, Cai JQ, Huang LH, Yang SL, Ma YJ, Fu YF, Xu XM, Tan JM. Mesenchymal Stem Cells Promote Islet Survival In Vitro and Function In Vivo, CellR4 2013; 1:e382. [Google Scholar]
- [29].Karaoz E, Genc ZS, Demircan PC, Aksoy A, Duruksu G. Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell death dis 2010; 1:e36; PMID: 21364643; http://dx.doi.org/ 10.1038/cddis.2010.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jung EJ, Kim SC, Wee YM, Kim YH, Choi MY, Jeong SH, Lee J, Lim DG, Han DJ. Bone marrow-derived mesenchymal stromal cells support rat pancreatic islet survival and insulin secretory function in vitro. Cytotherapy 2011; 13:19-29; PMID: 21142900; http://dx.doi.org/ 10.3109/14653249.2010.518608 [DOI] [PubMed] [Google Scholar]
- [31].Rackham CL, Dhadda PK, Chagastelles PC, Simpson SJ, Dattani AA, Bowe JE, Jones PM, King AJ. Pre-culturing islets with mesenchymal stromal cells using a direct contact configuration is beneficial for transplantation outcome in diabetic mice. Cytotherapy 2013; 15:449-459; PMID: 23321626; http://dx.doi.org/ 10.1016/j.jcyt.2012.11.008 [DOI] [PubMed] [Google Scholar]
- [32].Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells 2007; 25:2896-2902; PMID: 17901396; http://dx.doi.org/ 10.1634/stemcells.2007-0637 [DOI] [PubMed] [Google Scholar]
- [33].Yamada S, Shimada M, Utsunomiya T, Ikemoto T, Saito Y, Morine Y, Imura S, Mori H, Arakawa Y, Kanamoto M, et al.. Trophic effect of adipose tissue-derived stem cells on porcine islet cells. J Surg Res 2014; 187:667-672; PMID: 24238974; http://dx.doi.org/ 10.1016/j.jss.2013.10.031 [DOI] [PubMed] [Google Scholar]
- [34].Rackham CL, Vargas AE, Hawkes RG, Amisten S, Persaud SJ, Austin ALF, King AJF, Jones PM. Annexin A1 is a key modulator of mesenchymal stromal cell-mediated improvements in islet function. Diabetes. 2016; 65:129-139. PMID: 26470781 [DOI] [PubMed] [Google Scholar]
- [35].Kono TM, Sims EK, Moss DR, Yamamoto W, Ahn G, Diamond J, Tong X, Day KH, Territo PR, et al.. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: Analysis of hASC-derived paracrine effectors. Stem Cells 2014; 32:1831-1842; PMID: 24519994; http://dx.doi.org/ 10.1002/stem.1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Scuteri A, Donzelli E, Rodriguez-Menendez V, Ravasi M, Monfrini M, Bonandrini B, Figliuzzi M, Remuzzi A, Tredici G. A double mechanism for the mesenchymal stem cells' positive effect on pancreatic islets. PLoS ONE. 2014; 9 e84309; PMID: 24416216; http://dx.doi.org/ 10.1371/journal.pone.0084309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lu Y, Jin X, Chen Y, Li S, Yuan Y, Mai G, Tian B, Long D, Zhang J, Zeng L, et al.. Mesenchymal stem cells protect islets from hypoxia/reoxygenation-induced injury. Cell Biochem Funct 2010; 28:637-643; PMID: 21061411; http://dx.doi.org/ 10.1002/cbf.1701 [DOI] [PubMed] [Google Scholar]
- [38].Duprez IR, Johansson U, Nilsson B, Korsgren O, Magnusson PU. Preparatory studies of composite mesenchymal stem cell islets for application in intraportal islet transplantation. Ups J Med Sci 2011; 116:8-17; PMID: 21050099; http://dx.doi.org/ 10.3109/03009734.2010.524320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yoshimatsu G, Sakata N, Tsuchiya H, Ishida M, Motoi F, Egawa S, Sumi S, Goto M, Unno M. Development of polyvinyl alcohol bioartificial pancreas with rat islets and mesenchymal stem cells. Transplant Proc. 2013; 45:1875-1880; PMID: 23769061; http://dx.doi.org/ 10.1016/j.transproceed.2013.01.043 [DOI] [PubMed] [Google Scholar]
- [40].Yoshimatsu G, Sakata N, Tsuchiya H, Minowa T, Takemura T, Morita H, Hata T, Fukase M, Aoki T, Ishida M, et al.. The co-transplantation of bone marrow derived mesenchymal stem cells reduced inflammation in intramuscular islet transplantation. PLoS One 2015; 10:e0117561; PMID: 25679812; http://dx.doi.org/ 10.1371/journal.pone.0117561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lin P, Chen L, Li D, Yang N, Sun Y, Xu Y. Dynamic analysis of bone marrow mesenchymal stem cells migrating to pancreatic islets using coculture microfluidic chips: An accelerated migrating rate and better survival of pancreatic islets were revealed. Neuroendocrinol Lett 2009; 30:204-208. PMID: 19675523 [PubMed] [Google Scholar]
- [42].Karaoz E, Ayhan S, Okcu A, Aksoy A, Bayazit G, Osman Gurol A, Duruksu G. Bone marrow-derived mesenchymal stem cells co-cultured with pancreatic islets display (beta) cell plasticity. J Tissue Eng Regen Med 2011; 5:491-500; PMID: 21604384; http://dx.doi.org/ 10.1002/term.342 [DOI] [PubMed] [Google Scholar]
- [43].Davis NE, Beenken-Rothkopf LN, Mirsoian A, Kojic N, Kaplan DL, Barron AE, Fontaine MJ. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials 2012; 33:6691-6697; PMID: 22766242; http://dx.doi.org/ 10.1016/j.biomaterials.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kerby A, Jones ES, Jones PM, King AJ. Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice. Cytotherapy 2013; 15:192-200; PMID: 23321331; http://dx.doi.org/ 10.1016/j.jcyt.2012.10.018 [DOI] [PubMed] [Google Scholar]
- [45].Jun Y, Kang AR, Lee JS, Park SJ, Lee DY, Moon SH, Lee SH. Microchip-based engineering of super-pancreatic islets supported by adipose-derived stem cells. Biomaterials 2014; 35:4815-4826; PMID: 24636217; http://dx.doi.org/ 10.1016/j.biomaterials.2014.02.045 [DOI] [PubMed] [Google Scholar]
- [46].Rackham CL, Dhadda PK, Le Lay AM, King AJF, Jones PM. Pre-culturing islets with adipose-derived mesenchymal stromal cells is an effective strategy for improving transplantation efficiency at the clinically preferred intraportal site. Cell Medicine 2014; 7:37-47; PMID:4733844; http://dx.doi.org/25993680 10.3727/215517914x680047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al.. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64:401-406; http://dx.doi.org/ 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- [48].Rheinheimer J, Bauer AC, Silveiro SP, Estivalet AA, Boucas AP, Rosa AR, de Souza BM, de Oliveira FS, Cruz LA, Brondani LA, et al.. Human pancreatic islet transplantation: an update and description of the establishment of a pancreatic islet isolation laboratory. Arch Endocrinol Metab 2015;59:161-170; PMID: 25993680; http://dx.doi.org/ 10.1590/2359-3997000000030 [DOI] [PubMed] [Google Scholar]
- [49].Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells 2015; 8: 54-68; PMID: 26019755; http://dx.doi.org/ 10.15283/ijsc.2015.8.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet 2001; 29:306-309; PMID: 11600885; http://dx.doi.org/ 10.1038/ng749 [DOI] [PubMed] [Google Scholar]
- [51].Yeung TY, Seeberger KL, Kin T, Adesida A, Jomha N, Shapiro AM, Korbutt GS. Human mesenchymal stem cells protect human islets from pro-inflam-matory cytokines. PLoS One. 2012; 7:e38189; PMID: 22666480; http://dx.doi.org/ 10.1371/journal.pone.0038189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535; PMID: 19622551; http://dx.doi.org/ 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629-634; PMID: 9310563; http://dx.doi.org/ 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Higgins JP, Thompson SG, Deeks JJ, Altman D. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557-560; PMID: 12958120; http://dx.doi.org/ 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539-1558; PMID: 12111919; http://dx.doi.org/ 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- [56].Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Soft 2010; 36; http://dx.doi.org/ 10.18637/jss.v036.i03 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


