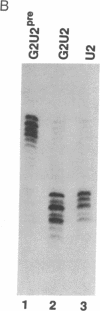
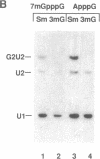
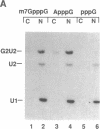
Abstract
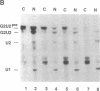
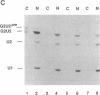
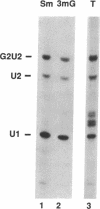
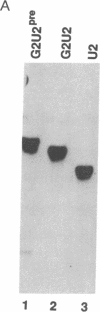
We present a method for studying RNA processing and ribonucleoprotein assembly in vivo, by using RNA synthesized in vitro. SP6-transcribed 32P-labeled U2 small nuclear RNA precursor molecules were introduced into cultured human 293 cells by calcium phosphate-mediated uptake, as in standard DNA transfection experiments. RNase protection mapping demonstrated that the introduced pre-U2 RNA underwent accurate 3' end processing. The introduced U2 RNA was assembled into ribonucleoprotein particles that reacted with an antibody specific for proteins known to be associated with the U2 small nuclear ribonucleoprotein particle. The 3' end-processed, ribonucleoprotein-assembled U2 RNA accumulated in the nuclear fraction. When pre-U2 RNA with a 7-methylguanosine group at the 5' end was introduced into cells, it underwent conversion to a 2,2,7-trimethylguanosine cap structure, a characteristic feature of the U-small nuclear RNAs. Pre-U2 RNA introduced with an adenosine cap (Ap-ppG) also underwent processing, small nuclear ribonucleoprotein assembly, and nuclear accumulation, establishing that a methylated guanosine cap structure is not required for these steps in U2 small nuclear ribonucleoprotein biosynthesis. Beyond its demonstrated usefulness in the study of small nuclear ribonucleoprotein biosynthesis, RNA transfection may be of general applicability to the investigation of eukaryotic RNA processing in vivo and may also offer opportunities for introducing therapeutically targeted RNAs (ribozymes or antisense RNA) into cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C. Transient gene expression control: effects of transfected DNA stability and trans-activation by viral early proteins. Mol Cell Biol. 1985 May;5(5):1034–1042. doi: 10.1128/mcb.5.5.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond V. C., Wold B. Poly-L-ornithine-mediated transformation of mammalian cells. Mol Cell Biol. 1987 Jun;7(6):2286–2293. doi: 10.1128/mcb.7.6.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M., Lienhard S., Parisot R. F. Intracellular transport of microinjected 5S and small nuclear RNAs. Nature. 1982 Feb 18;295(5850):572–577. doi: 10.1038/295572a0. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L. Short-lived, small RNAs in the cytoplasm of HeLa cells. Cell. 1974 Sep;3(1):11–14. doi: 10.1016/0092-8674(74)90031-2. [DOI] [PubMed] [Google Scholar]
- Feeney R. J., Sauterer R. A., Feeney J. L., Zieve G. W. Cytoplasmic assembly and nuclear accumulation of mature small nuclear ribonucleoprotein particles. J Biol Chem. 1989 Apr 5;264(10):5776–5783. [PubMed] [Google Scholar]
- Fisher D. E., Conner G. E., Reeves W. H., Wisniewolski R., Blobel G. Small nuclear ribonucleoprotein particle assembly in vivo: demonstration of a 6S RNA-free core precursor and posttranslational modification. Cell. 1985 Oct;42(3):751–758. doi: 10.1016/0092-8674(85)90271-5. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A. M., Patton J. R., Pederson T. U2 small nuclear RNP assembly in vitro. Nucleic Acids Res. 1989 Jun 26;17(12):4817–4828. doi: 10.1093/nar/17.12.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A. M., Pederson T. Accurate and efficient 3' processing of U2 small nuclear RNA precursor in a fractionated cytoplasmic extract. Mol Cell Biol. 1987 Sep;7(9):3131–3137. doi: 10.1128/mcb.7.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel G. R., Maser R. L., Calvet J. P., Pederson T. U6 small nuclear RNA is transcribed by RNA polymerase III. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8575–8579. doi: 10.1073/pnas.83.22.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrmann R., Appel B., Bringmann P., Rinke J., Reuter R., Rothe S., Bald R. Isolation and characterization of rabbit anti-m3 2,2,7G antibodies. Nucleic Acids Res. 1982 Nov 25;10(22):7103–7113. doi: 10.1093/nar/10.22.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Kunkel G. R., Pederson T. Precursors of U4 small nuclear RNA. J Cell Biol. 1984 Sep;99(3):1140–1144. doi: 10.1083/jcb.99.3.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Eukaryotic small ribonucleoproteins. Anti-La human autoantibodies react with U1 RNA-protein complexes. J Biol Chem. 1984 Feb 10;259(3):1929–1933. [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Intracellular site of U1 small nuclear RNA processing and ribonucleoprotein assembly. J Cell Biol. 1984 Jan;98(1):188–192. doi: 10.1083/jcb.98.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. W., Felgner P. L., Verma I. M. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986 Sep 12;46(6):905–911. doi: 10.1016/0092-8674(86)90072-3. [DOI] [PubMed] [Google Scholar]
- Mroczkowski B., Dym H. P., Siegel E. J., Heywood S. M. Uptake and utilization of mRNA by myogenic cells in culture. J Cell Biol. 1980 Oct;87(1):65–71. doi: 10.1083/jcb.87.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe S. H. Antisense RNA inhibits splicing of pre-mRNA in vitro. EMBO J. 1988 Aug;7(8):2523–2532. doi: 10.1002/j.1460-2075.1988.tb03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. R., Patterson R. J., Pederson T. Reconstitution of the U1 small nuclear ribonucleoprotein particle. Mol Cell Biol. 1987 Nov;7(11):4030–4037. doi: 10.1128/mcb.7.11.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson I., Hinterberger M., Mimori T., Gottlieb E., Steitz J. A. The structure of mammalian small nuclear ribonucleoproteins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984 May 10;259(9):5907–5914. [PubMed] [Google Scholar]
- Reddy R., Henning D., Das G., Harless M., Wright D. The capped U6 small nuclear RNA is transcribed by RNA polymerase III. J Biol Chem. 1987 Jan 5;262(1):75–81. [PubMed] [Google Scholar]
- Sauterer R. A., Feeney R. J., Zieve G. W. Cytoplasmic assembly of snRNP particles from stored proteins and newly transcribed snRNA's in L929 mouse fibroblasts. Exp Cell Res. 1988 Jun;176(2):344–359. doi: 10.1016/0014-4827(88)90336-9. [DOI] [PubMed] [Google Scholar]
- Tani T., Watanabe-Nagasu N., Okada N., Ohshima Y. Molecular cloning and characterization of a gene for rat U2 small nuclear RNA. J Mol Biol. 1983 Aug 15;168(3):579–594. doi: 10.1016/s0022-2836(83)80303-9. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Wieben E. D., Nenninger J. M., Pederson T. Ribonucleoprotein organization of eukaryotic RNA. XXXII. U2 small nuclear RNA precursors and their accurate 3' processing in vitro as ribonucleoprotein particles. J Mol Biol. 1985 May 5;183(1):69–78. doi: 10.1016/0022-2836(85)90281-5. [DOI] [PubMed] [Google Scholar]
- Wieben E. D., Pederson T. Small nuclear ribonucleoproteins of Drosophila: identification of U1 RNA-associated proteins and their behavior during heat shock. Mol Cell Biol. 1982 Aug;2(8):914–920. doi: 10.1128/mcb.2.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuo C. Y., Ares M., Jr, Weiner A. M. Sequences required for 3' end formation of human U2 small nuclear RNA. Cell. 1985 Aug;42(1):193–202. doi: 10.1016/s0092-8674(85)80115-x. [DOI] [PubMed] [Google Scholar]
- Zieve G. W., Sauterer R. A., Feeney R. J. Newly synthesized small nuclear RNAs appear transiently in the cytoplasm. J Mol Biol. 1988 Jan 20;199(2):259–267. doi: 10.1016/0022-2836(88)90312-9. [DOI] [PubMed] [Google Scholar]