Abstract
Background: Published literature has demonstrated commercially available premix vancomycin (5 mg/mL) and piperacillin-tazobactam (67.5 mg/mL) as physically compatible via simulated Y-site methodology. Compatibility via actual Y-site infusion has yet to be established.
Objective: To assess and compare the compatibility of commercially available premix concentrations of vancomycin and piperacillin-tazobactam via simulated and actual Y-site evaluation.
Methods: Vancomycin and piperacillin-tazobactam were tested using simulated and actual Y-site infusion methodologies. Simulated Y-site compatibility was performed using previously published methods via visual inspection, turbidity evaluation, and pH evaluation. Evaluation occurred immediately, 60 minutes, 120 minutes, and 240 minutes following mixing for each mixture and control. Mixtures were considered physically incompatible if there was visual evidence of precipitation or haze, an absorbance value was greater than 0.01 A, or an absolute change of 1.0 pH unit occurred. Actual Y-site infusion was simulated to mirror antibiotic infusion in the clinical setting by nursing personnel using smart pumps and intravenous tubing.
Results: No evidence of physical incompatibility was observed during simulated Y-site testing via visual inspection, turbidity assessment, and pH evaluation. Conversely, physical incompatibility was observed to the unaided eye within 2 minutes during actual Y-site infusion.
Conclusions: Despite observed compatibility between vancomycin and piperacillin-tazobactam via simulated Y-site testing, visual evidence of physical incompatibility was observed during actual Y-site infusion. This poses a potential compromise to patient safety if these antibiotics are administered simultaneously in the clinical setting. Actual Y-site testing should be performed prior to clinical adoption of compatibility studies that are based solely on simulated methodologies.
Keywords: incompatibility, piperacillin-tazobactam, premix antibiotics, vancomycin, Y-site compatibility
The combination of vancomycin hydrochloride and piperacillin-tazobactam is a commonly utilized antibiotic regimen for patients who require broad-spectrum antibiotic coverage, including patients with sepsis.1 Vancomycin and piperacillin-tazobactam are commercially available as premix formulations in dextrose 5% water (D5W) with concentrations of 5 mg/mL and 67.5 mg/mL, respectively.2,3 These premix formulations are theoretically advantageous because they minimize the preparation time required by pharmacy personnel and are able to be stored on medical floors in automated dispensing cabinets. Simultaneous infusion of these therapies is desirable in the clinical setting in order to reduce time to antibiotic administration and potentially improve patient outcomes.4–6
Vancomycin and piperacillin-tazobactam have historically been considered to be Y-site incompatible at various concentrations.7–10 However, recent published data suggest that vancomycin and piperacillin-tazobactam are physically compatible, particularly at commercially available premix formulation concentrations.11 Given the logistical advantages of simultaneous administration, some institutions may have considered allowing concurrent Y-site administration for these agents in the clinical setting based on these reports. However, anecdotal reports from our institution suggest vancomycin and piperacillin-tazobactam are visually incompatible via Y-site infusion at premix concentrations. The discordance between the published data and our clinical experience may be related to the methodology utilized in compatibility testing. Most compatibility studies mix investigated medications in a 1:1 mixture within a test tube and test physical compatibility using simulated Y-site compatibility methodology including visual inspection, turbidity assessment, and pH evaluation.7–11 This procedure does not account for physiochemical interactions that may occur during actual administration, such as the interaction between medications and intravenous (IV) tubing. Furthermore, previous literature has suggested that some medications show evidence of physical incompatibility during actual Y-site administration.12–14 It is therefore prudent to assess the compatibility of these medications during both simulated and actual Y-site administration.
We sought to confirm the physical compatibility of premix vancomycin 5 mg/mL and piperacillin-tazobactam 67.5 mg/mL in D5W via simulated Y-site infusion. Additionally, we also sought to assess the compatibility of these antibiotics during actual Y-site infusion. Herein, we report our compatibility findings of premix vancomycin 5 mg/mL and piperacillin-tazobactam 67.5 mg/mL in D5W during simulated and actual Y-site infusion.
METHODS
Commercially available premix vancomycina 5 mg/mL in D5W and premix piperacillin-tazobactamb 67.5 mg/mL in D5W were obtained from hospital stock. Piperacillin-tazobactam content was expressed in terms of the combination product. Expiration dates of these antibiotics were reviewed to ensure expired products were not utilized. Premix preparations were thawed per manufacturer recommendations. Once thawed, vancomycin and piperacillin-tazobactam were assigned a 30-day and 14-day expiration date, respectively.2,3 All preparations were stored at 4°C until the morning of the experiment and brought to room temperature (21°C–25°C) prior to analysis. All experiments were completed at least twice to enhance the validity and accuracy of simulated Y-site testing.
The methods used to simulate Y-site infusion were similar to that reported in previous publications.15–20 During Y-site infusion, drugs were mixed in a 1:1 ratio.15 To simulate this, 5 samples were created containing 2 mL of premix vancomycin 5 mg/mL in D5W with 2 mL of premix piperacillin-tazobactam 67.5 mg/mL in D5W. Control solutions containing either 2 mL of vancomycin 5 mg/mL in D5W or piperacillin-tazobactam 67.5 mg/mL in D5W were also created. All samples were prepared in glass test tubes. Test samples were prepared by first adding vancomycin to the glass test tubes and then rapidly adding piperacillin-tazobactam to simulate a simultaneously delivered 1:1 mixture.
All samples were initially assessed for evidence of visual incompatibility using the unaided eye. If evidence of visual incompatibility was detected, the experiment was terminated for that sample. Samples without evidence of visual incompatibility to the unaided eye were then assessed using enhanced visual inspection, turbidity measurements in the visible light wavelength range, and pH evaluation. Assessments were performed at 0, 60, 120, and 240 minutes after mixing for each mixture and control. All visual assessments were independently completed by 2 separate reviewers and compared to control solutions. Visual inspection was performed using an unaided eye, a high-intensity light source, and a magnifying glass against white and black backgrounds.16–18,21 Absorbance was measured at 660 nanometers (nm) using a laboratory grade scanning spectrophotometerc with a photometric accuracy of ± 0.005 A. This wavelength was selected based on previous publications and the known spectra of turbid samples.9,22 Based on the spectrophotometer's photometric accuracy, absorbance <0.005 A was reported as <0.005 A. Sample pH was assessed using a pH meterd. All laboratory equipment was calibrated and checked for accuracy by expert personnel prior to use. Based on previously published literature, incompatibility was defined as the presence of haze, particulate formation, gas evolution, color change, or precipitation on visual inspection, ≥1 pH unit change over the 4-hour course of the experiment, or any absorbance of >0.01 A.7–11,23–25
To simulate actual Y-site infusion, we sought to replicate the infusion process conducted by nursing personnel in the clinical setting. All actual Y-site infusions were completed in duplicate to enhance the validity and accuracy of this study. Three Alaris smart pumpse, each with 2 infusion channels, were obtained. Primary Smartsite infusion setsf with secondary sites (Y-site) were used in each channel. Each infusion set was nonlatex, non-di(2-ethylhexyl)phthalate (DEHP), single-use lumens. Each respective lumen was primed with either vancomycin 5 mg/mL in D5W or piperacillin-tazobactam 67.5 mg/mL in D5W to simulate our institution's clinical practice. Vancomycin 5 mg/mL in D5W and piperacillin-tazobactam 67.5 mg/mL in D5W were run as independent, primary infusions on the first smart pump to serve as negative controls. Vancomycin 5 mg/mL in D5W and piperacillin-tazobactam 67.5 mg/mL in D5W were run on the second and third smart pump and were connected via a secondary site (Y-site) below the infusion channels. Vancomycin and piperacillin-tazobactam were run at a rate of 200 mL/h and 12.5 mL/h, respectively, to mirror current clinical practice. All infusions were run into sterile glass collection vialsg. To control for possible interactions with the infusion pump and IV tubing, additional samples containing vancomycin 5 mg/mL in D5W, piperacillin-tazobactam 67.5 mg/mL in D5W, and a 1:1 combination of vancomycin 5 mg/mL and piperacillin-tazobactam 67.5 mg/mL in D5W were created by directly injecting the antibiotic solutions into separate sterile glass vials. Absorbance and pH analyses were not performed for time zero of the solutions during actual Y-site infusion.
RESULTS
Simulated Y-site Infusion
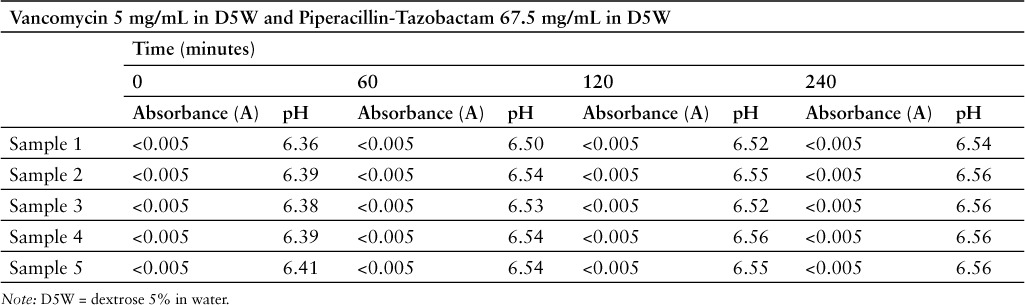
No evidence of physical incompatibility was observed over the 4-hour study period. Mixtures appeared similar to control solutions at all time points, and there was no identified evidence of haze, discoloration, or precipitation. Visual differences were not identified using the unaided eye, a high-intensity light source, or a magnifying glass. All samples appeared similar to controls against white and black backgrounds.
Results of the absorbance and pH measurements are represented in Table 1. Again, no evidence of incompatibility was observed over the 4-hour study period. The largest pH change observed over the 4-hour course of the experiment was 0.20 (6.36 vs 6.46), which is well below the established cutoff change of ≥1 pH unit. No sample had more than a 3% absolute change in pH, and there was less than 4% variability in the experiment as a whole. All absorbance readings were below the level of the spectrophotometer's photometric accuracy (± 0.005 A) and mixtures were, therefore, similar to baseline controls.
Table 1.
Absorbance and pH measurements during simulated Y-site evaluation
Actual Y-site Infusion
For actual Y-site infusion, visual evidence of physical incompatibility was noted within the first 2 minutes of all infusions. In both circumstances, a white, opaque, turbid liquid formed in the sterile glass vials collecting the vancomycin and piperacillin-tazobactam mixture. This was visibly different from the vancomycin and piperacillin-tazobactam controls, which were run as independent, primary infusions. These control solutions appeared clear, and there was no evidence of precipitation or physical incompatibility. Additionally, the control containing the vancomycin and piperacillin-tazobactam mixture, prepared via direct injection into sterile glass vials, was also clear and showed no evidence of physical incompatibility. Visual inspection of the IV tubing revealed evidence of physical incompatibility below the Y-site connector within the first 30 minutes for all infusions. This is described as a white precipitant, similar to the one noted above, in addition to crystal particulate matter. No evidence of incompatibility was noted in the tubing sets of the vancomycin and piperacillin-tazobactam control infusions. Absorbance and pH analyses were not performed for experimental samples because gross visual evidence of physical incompatibility was quickly observed.
DISCUSSION
We observed clear evidence of physical incompatibility, including precipitation below the Y-site connector, when vancomycin 5 mg/mL in D5W was given as an actual Y-site infusion with piperacillin-tazobactam 67.5 mg/mL in D5W. This white precipitant was consistent with previous reports of vancomycin and piperacillin-tazobactam incompatibility.8,9 No evidence of physical incompatibility was noted when standard procedures were used to simulate Y-site infusion. The mechanism of this discrepancy is unclear, but we believe it may likely be secondary to an interaction between the IV tubing and the combined mixture of vancomycin and piperacillin-tazobactam. This hypothesis is supported by 2 observations. First, there was no evidence of physical incompatibility when the medications were run as primary, independent infusions, indicating that the combined mixture is necessary for precipitant formation. Second, no evidence of incompatibility was observed when the medications were combined via direct injection into a sterile glass vial. This indicates that not only the combined mixture is necessary to cause incompatibility, but also the combined infusion through the IV tubing.
Based on the available literature, Y-site compatibility of vancomycin and piperacillin-tazobactam appears to be concentration-dependent.7–11 These studies used standard methods to simulate Y-site infusion, mixing vancomycin and piperacillin-tazobactam in a 1:1 ratio in glass test tubes. Several of these studies showed these medications were compatible at a variety of concentrations. Leung and colleagues found vancomycin 4 mg/mL in normal saline (NS) and piperacillin-tazobactam 33.75 to 45 mg/mL in NS were physically compatible.7 Wade and colleagues found that vancomycin 4 mg/mL in D5W was physically compatible with piperacillin-tazobactam 40 mg/mL in D5W.8 O'Donnell and colleagues found that vancomycin 4 to 7 mg/mL in D5W or NS was physically compatible with piperacillin-tazobactam 33.75 to 90 mg/mL in D5W or NS.11 Based on these studies, it would appear that vancomycin and piperacillin-tazobactam are physically compatible at a variety of concentrations. However, none of these studies assessed actual Y-site compatibility, using a smart pump and IV tubing. Our study showed no evidence of physical incompatibility via stimulated Y-site infusion, which is consistent with the findings by O'Donnell and colleagues at commercially available premix concentrations.11 Conversely, physical incompatibility was evident within minutes of initiating actual Y-site infusion. Therefore, we do not believe the results of these previous studies can be applied to clinical practice.
This is the first report to our knowledge attributing an incompatibility to IV tubing, and it calls into question our current practices for determining Y-site compatibility. Most Y-site compatibility studies establish the absence or presence of incompatibility in a test tube. Any evidence of incompatibility would strongly suggest a similar reaction in a clinical practice. However, the absence of incompatibility from this method cannot absolutely confirm the absence of incompatibility in clinical practice given the discordance between our simulated and actual Y-site results, as the simulated Y-site infusion method does not control for commonly encountered variables such as IV tubing.
Though less common, some studies have utilized actual Y-site infusion to assess the compatibility of medications. Condie and colleagues assessed the physical compatibility of IV caspofungin and 31 commonly utilized medications.12 Caspofungin was delivered through a polyvinyl chloride (PVC) tubing set. All other medications were infused through a secondary site. The authors used visual inspection and microscopy to assess for physical compatibility. Hutchings and colleagues assessed the physical compatibility of cefmetazole sodium and 34 commonly used drugs and solutions.14 Cefmetazole sodium was delivered as a primary injection and all other medications were delivered via IV push, IV infusion, or syringe pump using the secondary site. The authors used visual methods and microscopy to assess for physical compatibility. Based on our results, we would encourage clinicians to consider performing actual Y-site infusion in addition to simulated Y-site infusion when assessing drug compatibility.
Our study has several important limitations to consider. First, the clinical significance of the observed incompatibility in the sterile glass vial is unclear. More than likely, these medications would rapidly distribute, and it is possible that this incompatibility would not occur when administered to the patient. However, these samples contain an approximate 1:1 mixture of vancomycin and piperacillin-tazobactam. This approximates the 1:1 mixtures collected in many compatibility studies in glass test tubes. Evidence of incompatibility within minutes of mixing would constitute evidence of incompatibility. Additionally, infusions are frequently stopped and restarted in clinical practice. These mixtures reflected the static combination of vancomycin and piperacillin-tazobactam, and evidence of incompatibility in this state is concerning. Furthermore, the clinical relevance of incompatibility in the IV tubing is unquestionable. Second, we used commercially available, fixed concentrations of vancomycin and piperacillin-tazobactam premix formulations that were relevant to our institution. It is unclear if these results can be extrapolated to other concentrations and preparations. Third, we used common IV tubing specific to our hospital. It is unclear whether similar results would be observed if other types of IV tubing were used. Last, our results during actual Y-site infusion cannot definitively determine incompatibility risks when these therapies are infused directly into a patient in the clinical setting. However, it would be unethical to simultaneously administer these 2 antibiotics at these concentrations to a patient in the clinical setting after our observed results of actual Y-site evaluation.
CONCLUSION
Commercially available premix formulations of vancomycin 5 mg/mL in D5W combined with piperacillin-tazobactam 67.5 mg/mL in D5W via simulated Y-site infusion demonstrated no evidence of physical incompatibility. However, actual Y-site infusion of the 2 antibiotics at these fixed concentrations resulted in the formation of a white precipitate suggestive of physical incompatibility. Our study clearly demonstrates discordance between simulated and actual Y-site evaluation. Furthermore, our results suggest the importance of including actual Y-site evaluation as a component of future compatibility studies. Clinicians are encouraged to confirm our compatibility results before implementing simultaneous infusions of vancomycin and piperacillin-tazobactam at commercially available premix concentrations in the clinical practice setting.
ACKNOWLEDGMENTS
The authors report no conflicts of interest.
Footnotes
a Vancomycin injection in Galaxy plastic container, Baxter Healthcare Corporation, Deerfield, IL, lot NC097147.
b Piperacillin-Tazobactam injection in Galaxy plastic container, Pfizer, Philadelphia, PA, lot LN098632.
c Beckman DU-640B UV-Visible, Scanning Spectrophotometer, Fullerton, CA
d Markson Microcomputer pH Meter (model 6200), Honolulu, HI
e Alaris Smart Pump Module, Carefusion Corporation, San Diego, CA
f Smartsite Infusion Set, Carefusion Corporation, San Diego, CA
g Sterile 30 mL Empty Vial, Hospira, Lake Forest, IL, lot 51044EV
REFERENCES
- 1. Pakyz AL, MacDougall C, Oinonen M, . et al. Trends in antibacterial use in US academic health centers. Arch Intern Med. 2008; 168: 2254– 2260. [DOI] [PubMed] [Google Scholar]
- 2. Zosyn (piperacillin sodium-tazobactam) [package insert]. Philadelphia, PA: Pfizer; May 2012. [Google Scholar]
- 3. Vancomycin hydrochloride [package insert]. Lake Forest, IL: Hospira; November 2008. [Google Scholar]
- 4. Kumar A, Roberts D, Wood KE, . et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006; 34( 6): 1589– 1596. [DOI] [PubMed] [Google Scholar]
- 5. Dellinger RP, Levy MM, Rhodes A, . et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intens Care Med. 2013; 39( 2): 165– 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok K, Christian MD, Nelson S, . et al. Time to infusion of antibiotics among inpatients with severe sepsis or septic shock. Can J Hosp Pharm. 2014; 67( 3): 213– 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung E, Venkatesan N, Ly SC, . et al. Physical compatibility of vancomycin and piperacillin sodium-tazobactam at concentrations typically used during prolonged infusions. Am J Health Syst Pharm. 2013; 70( 13): 1163– 1166. [DOI] [PubMed] [Google Scholar]
- 8. Wade J, Cooper M, Ragan R.. Simulated Y-site compatibility of vancomycin and piperacillin-tazobactam. Hosp Pharm. 2015; 50( 5): 376– 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nichols KR, Demarco MW, Vertin MD, . et al. Y-site compatibility of vancomycin and piperacillin/tazobactam at commonly utilized pediatric concentrations. Hosp Pharm. 2013; 48( 1): 44– 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trissel LA, Gilbert DL, Martinez JF.. Concentration dependency of vancomycin hydrochloride compatibility with beta-lactam antibiotics during simulated Y-site infusion. Hosp Pharm. 1998; 33: 1515– 1522. [Google Scholar]
- 11. O'Donnell JN, Venkatesan N, Manek M, . et al. Visual and absorbance analyses of admixtures containing vancomycin and piperacillin-tazobactam at commonly used concentrations. Am J Health Syst Pharm. 2016; 73( 4): 241– 246. [DOI] [PubMed] [Google Scholar]
- 12. Condie CK, Tyler LS, Barker B, . et al. Visual compatibility of caspofungin acetate with commonly used drugs during simulated Y-site delivery. Am J Health Syst Pharm. 2008; 65( 5): 454– 457. [DOI] [PubMed] [Google Scholar]
- 13. Najari Z, Rusho WJ.. Compatibility of commonly used bone marrow transplant drugs during Y-site delivery. Am J Health Syst Pharm. 1997; 54( 2): 181– 184. [DOI] [PubMed] [Google Scholar]
- 14. Hutchings SR, Rusho WJ, Tyler LS.. Compatibility of cefmetazole sodium with commonly used drugs during Y-site delivery. Am J Health Syst Pharm. 1996; 53( 18): 2185– 2188. [DOI] [PubMed] [Google Scholar]
- 15. Allen LV Jr, Levinson RS, Phisutsinthop D.. Compatibility of various admixtures with secondary additives at Y-injection sites of intravenous infusion sets. Am J Health Syst Pharm. 1977; 34( 9): 939– 943. [PubMed] [Google Scholar]
- 16. Newton DW. Drug incompatibility chemistry. Am J Health Syst Pharm. 2009; 66( 4): 348– 357. [DOI] [PubMed] [Google Scholar]
- 17. Dasta JF, Hale KN, Stauffer GL, . et al. Comparison of visual and turbidimetric methods for determining short-term compatibility of intravenous critical-care drugs. Am J Health Syst Pharm. 1988; 45( 11): 2361– 2366. [PubMed] [Google Scholar]
- 18. Kohut J 3rd, Trissel LA, Leissing NC.. Don't ignore details of drug-compatibility reports. Am J Health Syst Pharm. 1996; 53( 19): 2339. [DOI] [PubMed] [Google Scholar]
- 19. Trissel LA, Flora KP.. Stability studies: Five years later. Am J Health Syst Pharm. 1988; 45( 7): 1569– 1571. [PubMed] [Google Scholar]
- 20. Trissel LA. Avoiding common flaws in stability and compatibility studies of injectable drugs. Am J Hosp Pharm. 1983; 40( 7): 1159– 1160. [PubMed] [Google Scholar]
- 21. Kanji S, Lam J, Johanson C, . et al. Systematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units. Crit Care Med. 2010; 38( 9): 1890– 1898. [DOI] [PubMed] [Google Scholar]
- 22. Nikolac N. Lipemia: Causes, interference mechanisms, detection and management. Biochemia Medica. 2014; 24( 1): 57– 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trissel LA. Handbook on Injectable Drugs. 17th ed. Bethesda, MD: American Society of Health-System Pharmacists; 2013. [Google Scholar]
- 24. Dotson B, Lynn S, Savakis K, . et al. Physical compatibility of 4% sodium citrate with selected antimicrobial agents. Am J Health Syst Pharm. 2010; 67( 14): 1195– 1198. [DOI] [PubMed] [Google Scholar]
- 25. Teibel HM, Knoderer CA, Nichols KR.. Compatibility of vancomycin and oxacillin during simulated Y-site delivery. Hosp Pharm. 2015; 50( 8): 710– 713. [DOI] [PMC free article] [PubMed] [Google Scholar]


