Abstract
It is debated whether exercise-induced ROS production is obligatory to cause adaptive response. It is also claimed that antioxidant treatment could eliminate the adaptive response, which appears to be systemic and reportedly reduces the incidence of a wide range of diseases. Here we suggest that if the antioxidant treatment occurs before the physiological function-ROS dose-response curve reaches peak level, the antioxidants can attenuate function. On the other hand, if the antioxidant treatment takes place after the summit of the bell-shaped dose response curve, antioxidant treatment would have beneficial effects on function. We suggest that the effects of antioxidant treatment are dependent on the intensity of exercise, since the adaptive response, which is multi pathway dependent, is strongly influenced by exercise intensity. It is further suggested that levels of ROS concentration are associated with peak physiological function and can be extended by physical fitness level and this could be the basis for exercise pre-conditioning. Physical inactivity, aging or pathological disorders increase the sensitivity to oxidative stress by altering the bell-shaped dose response curve.
Highlights
-
•
Exercise and reactive oxidative stress dose response can be described by bell shape curve.
-
•
Antioxidant supplementation can depress or enhance physiological function depending on timing.
-
•
Higher level of physical fitness is associated with greater tolerance to oxidative stress.
-
•
The health benefits of exercise is related to adaptive capacity to oxidative stress.
Graphical abstract
1. Introduction
The development of the genome for the genesis of humans has been associated with a physically active lifestyle. Radak et al. [51] have suggested that stone- age man used 4000 kcal for physical activity on a daily basis. It is well known that adaptive changes on the DNA sequence are very slow and therefore, it is not surprising that physical inactivity is a risk factor for a wide range of diseases. Indeed, physical inactivity- associated diseases cause a large burden on the healthcare system of many societies [16].
In accordance with these findings, there is a large body of evidence showing that regular exercise reduces the risk of life-style related diseases [29], [32], [5], [56], [65], increases mean life-span [12] and, significantly increases the quality of life, especially in the elderly [18], [9]. It is important to note that the effects of exercise are systemic and complex [44]. Regular exercise can beneficially affect brain function resulting in neurogenesis, elevated production of neurotrophic and growth factors, and improved capillarization [46]. Regular exercise increases the content of glucose transporter, GLUT4, enhances insulin sensitivity, and reduces the risk of the metabolic syndrome [54]. In addition, regular aerobic exercise beneficially affects the function of kidney, liver [39] and, of course, heart and skeletal muscle [36]. Moreover, regular exercise can even modify the function of the testis, [59] improving erectile dysfunction in physically active men [30]. Regular exercise effects the senses, diminishing the age- associated loss in hearing [23], and oxidant- induced degeneration in the eye [26]. As well, regular exercise has been shown to significantly prevent UV radiation- induced carcinogenesis in skin of mice [33] and to ameliorate kidney disease [60].
Overall, regular exercise-induced systemic adaptation is a powerful factor that decreases the incidence of a wide range of diseases. One can argue that present day regular exercise programs simply normalize the rate of physical activity to that point at which our ancestors lived generations ago. Before the technology revolution of the twentieth century, agriculture, transportation, and war, tested man's physical state. No one asked the questions, whether 16 h of daily physical work in the fields was a healthy endeavour or if moving from central Asia to Europe, on foot, could jeopardize life with the intensive production of reactive oxygen species (ROS). These types of physical activities were considered to be normal. Today, one thinks twice about going up to the six floor if the elevator is out of order.
It has long been known that ROS are important, necessary agents for cellular function and it is clear that they are controlling a number of physiological functions in skeletal muscle. Some years ago it was debated whether antioxidant treatment could attenuate or eliminate exercise-induced adaptive response in skeletal muscle. It was demonstrated that giving antioxidant vitamin C or C and E complex reduced PGC-1 a or NRF2 activation and the whole adaptive response was knocked out ([20], [21], [35], [38], [40], [53], [61] (Table 1.)). However, other investigators noted that the adaptive response is much more complex and regulated by multi protein pathways and signaling agents besides ROS, and also that antioxidant treatment did not eliminate the beneficial effects of exercise [13], [25], [28], [55], [58], [64].
Table 1.
The effects of antioxidant supplementation on exercise-induced adaptation.
Would it be possible that such an important physiological process as exercise-induced adaptation, which could be one of the driving forces of human evolution [31], [62], [8], could be eliminated by reduction of ROS production? Or are ROS so important that without an exercise-induced ROS production mitochondrial biogenesis, muscle hypertrophy, improved brain function, decreased incidence of life-style related diseases or regeneration would not occur? If one took 1000 mg of vitamin C per day and ran 10,000 m daily, would it be in vain? Would we be as prone to diseases as the physically untrained?
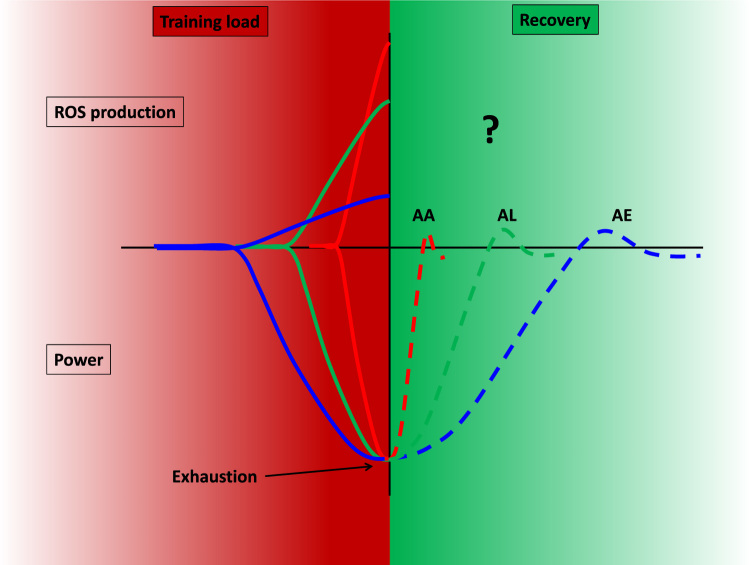
It is quite clear that the adaptive response to exercise depends on the intensity of the exercise, and the eventual ROS production, which is intensity dependent (Fig. 1). Exercise with very high intensity using ATP, CrP as the main energy sources, where fatigue is due to the accumulation of Pi, AMP, H+, ammonia and other metabolic products, is not normal practice. Recovery is very fast after anaerobic alactic dominant fatigue. When ATP is regenerated from mainly glycolytic pathways decreased pH and Na-K pump excitability levels could lead to fatigue, among other factors, but recovery within an hour. When aerobic exercise is used, one of the reasons for fatigue is the depletion of muscle glycogen. The recovery of this store takes days. High intensity exercise produces higher concentration of ROS then exercise with moderate intensity. However, the time course to normalize ROS levels is not really known. One of the reasons for this is the lack of proper methodology to detect very short lived ROS, but it is quite accepted that greater exercise intensity leads to greater production of ROS [41].
Fig. 1.
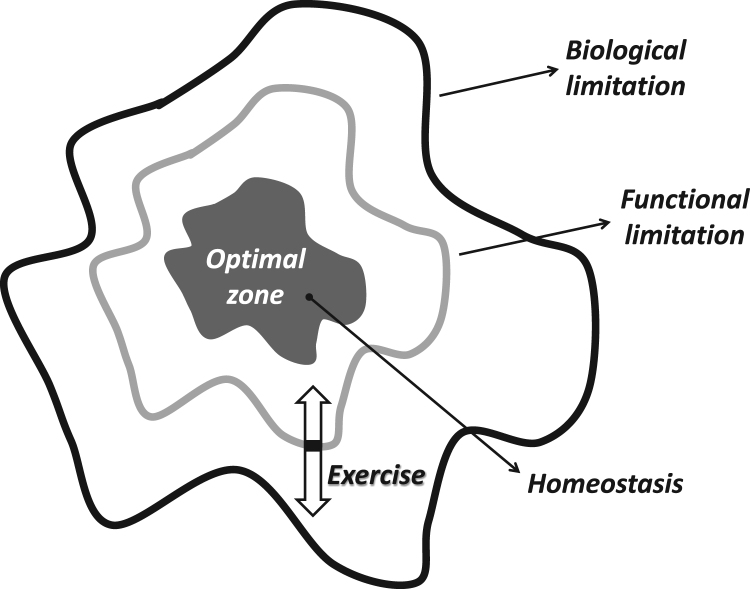
The hypothetical adaptive range. The middle of the graph represents the optimal zone of the dynamic homeostasis, while the outer line indicates the biological limitations, which cannot be reached without risk of death. The line, called functional limitation, shows the capacity of each individual and it is a mobile value. The functional/actual limit can be readily altered by exercise training. Aging decreases the rate of adaptive response, and the capacity to maintain homeostasis is decreasing, as demonstrated by the white arrows.
Would that mean that the same amount of antioxidants would act differently due to the intensity of the training? The contribution of signaling pathways to mitochondrial biogenesis, as an example, which could be driven by Ca++, CaMK, ROS, AMPK, PKD1, p38a [63], would be the same at different exercise intensities? In all likelihood, this is not the case [14], [34]. Hence, the same amount of antioxidant would affect the exercise-induced adaptation in a different fashion.
Many of the aforementioned questions do not have concise and exact answers. In this paper we put forward a hypothesis by which some of the questions can be answered and which may explain the different results of antioxidant treatments on exercise-induced adaptation. The phenomena behind the different responses could be due to the dynamic nature of hormesis-like dose response.
2. Exercise, hormesis and functional endpoints
The thesis of the hormesis theory is that biological systems respond to exposure to chemicals, toxins, and radiation, and that hormesis is denoted by a bell-shaped curve. In toxicology, hormesis is a dose response phenomenon characterized by a low dose of stimulation, and a high dose of inhibition, resulting in either a bell-shaped or an inverted U-shaped dose response curve, which is a non-monotonic response [10], [11], [15]. More than a decade ago we extended the hormesis theory to reactive oxygen species (ROS), which appear to plateau when modulated by aging or physical exercise [43]. We proposed that exercise modulates ROS and the effects can be described by the hormesis curve. We believe that regular exercise results in a typical bell-shaped hormesis curve, due to the regulation of adaptive systems. We have proposed that adaptation is dependent on the modulation of homeostasis. It is clear that homeostasis is a dynamic system with biological and functional/actual end-points. Biological endpoints are signified by the point at which the system collapses.
Functional/actual endpoints demark the limits of individual tolerance, which are naturally below biological endpoints and are dynamic, variable values. The distance between the optimal zone and biological end points represents the zone which can be targeted to induce adaptations to extend functional/actual endpoints. In the case of a high degree of adaptation, the distance between the biological endpoints and the functional endpoints can be narrowed. In other words, the distance between the optimal zone and functional/actual endpoints can be increased (Fig. 2). It can be assumed that a larger range between optimal zone and functional/actual endpoints represents greater adaptive capability and better tolerance against stressors.
Fig. 2.
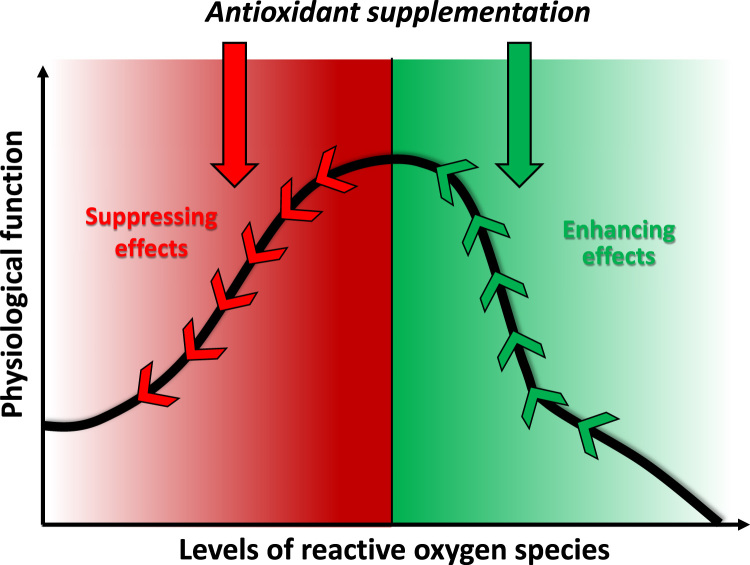
Supplementation of antioxidants before (-) the ROS levels reach the value associated with peak physiological function that can attenuate the beneficial effects of exercise. On the other hand antioxidant treatment, after (+) the period of maximum ROS-associated function can result in decreased appearance of fatigue and/or improved function.
Two examples will suffice to exemplify this point. The resting heart rate of untrained individuals is around 70 beats/min, while the maximal heart rate is approximately 220 minus the age of the individual. Therefore, the adaptive range is 130 beats for a twenty year old and just 50 beats for a centenarian. Well trained endurance runners, on the other hand, have a significant decrease in resting heart rate which could be as low as 35 beats per minute. If an individual has the same maximal heart rate as the twenty year old, trained and untrained, the adaptive range increases from 130 to 165 with the extension of the functional endpoints. Another example is lactic acid tolerance. Both trained and untrained individuals have a resting blood lactic acid level around 1.5 mM/L. If they run until exhaustion on a treadmill, untrained individuals stop when their lactic acid levels reach approximately 6–10 mM/L, while elite athletes can still continue until their lactic acid levels are over 20 mM/L.
Exercise-associated adaptive response is specific and depends on the intensity and duration of exercise. High intensity acute exercise produces different metabolic intermediates, such as lactic acid, ammonia, and adenosine monophosphate, than exercise of low intensity and high duration. Single bouts of aerobic exercise, on the other hand, could result in hypoglycemia, and a significant loss of glycogen content in skeletal muscle. Hence, the adaptive response to high intensity exercise would cause increased tolerance to lactic acid, a higher speed at the anaerobic threshold, and better elimination of ammonia, while regular aerobic exercise results in improved carbohydrate handling of the body, and increased levels of glycogen in skeletal muscle. These adaptations are brought about by regular exercise without which these adaptive responses would not occur. However, exercise above a certain threshold can cause mal-adaptations, which decrease the range of the biological and functional/actual adaptive zones. This is termed overtraining. Therefore, the two minimal endpoints of exercise-related dose response are physical inactivity and overtraining.
A similar type of adaptive response is found for exercise-induced ROS production. It is known that a single bout of exhaustive exercise results in elevated levels of lipid peroxidation, carbonylation of amino acid residues, and 8-Oxo-7,8 dihydroguanine (8-oxoG) in DNA [49]. On the other hand, when a single bout of exhaustive exercise is given to well trained subjects the body responds without a large elevation in oxidative damage [49]. In addition, regular exercise training- associated adaptation is a precondition against treatment with hydrogen peroxide, which causes a significant degree of damage in untrained subjects [48]. Moreover, when heart attack or stroke are simulated in untrained and trained animals, the infarct size is significantly smaller in trained groups [17], [7], showing that regular exercise acts as a pre-conditioning tool [48] by enhancing the adaptive zone, and by narrowing the theoretical distance between functional and biological endpoints. A great deal of evidence exists which suggests that regular exercise-induced adaptations to ROS handling, through redox signaling, including antioxidant and oxidative damage repair systems, significantly contribute to the health promoting effects of regular exercise. Another example is, that regular exercise decreases middle cerebral artery occlusion induced damage, by attenuation of ischemia/reperfusion mediated pathology via regulation of TLR4/NF-κB pathway [66]. Overall, it is suggested that regular exercise prevents a wide range of neurodegenerative diseases [3], including Alzheimer disease, as well [45] by the modulation of oxidant/antioxidant/repair systems.
3. Antioxidants and ROS-dependent hormesis with exercise
It is clear that moderate amounts of oxidizing agents, for example 150 μM H2O2, can increase the force generation of skeletal muscle, while larger doses (300 μM H2O2) result in a decline in force production [2]. It has also been demonstrated that administration of the antioxidant N-acetylcysteine (NAC) to humans decreased the fatigue-associated decline in force generation [52]. These findings demonstrate that elevated amounts of ROS cause fatigue (this is in association with the fact that high levels of ROS suppress force generation). On the other hand, there are a few recent reports suggesting that antioxidant supplementation is not always good, because it can suppress the adaptive response to exercise training [19], [20], [53]. However, it must be mentioned that this view is not fully accepted [25]. If the exercise generated ROS can be described by a bell-shaped curve, the opposing effects of antioxidant supplementation on ROS-induced adaptation can be easily understood. Fig. 3 displays our hypothesis that if the antioxidants are supplemented before the ROS reach levels for maximum adaptive response, the antioxidants would depress the physiological response. On the other hand, if the antioxidants are supplemented when the concentration of exercise-generated ROS is associated with a declining physiological response, the supplementation would result in enhanced performance and delayed fatigue. Indeed, the scientific database includes results for both situations, when the antioxidants were stimulating or suppressing. Our concept could be one possible explanation for these divergent results. Moreover, it must be mentioned that the so called maximal or optimal levels of ROS are dependent on many factors, such as age, history of the exposure to oxidative stress, the effectiveness of the endogenous antioxidant system, level of physical fitness etc. The question is, whether the different response to antioxidant treatment on physiological function, that we have just described, would cause different adaptive responses. It is well established that the cause of fatigue during exercise with different intensities is related to adaptation [4]. Therefore, alteration of the characteristics of fatigue most probably directly effects adaptive response. Exercise-induced adaptation increases the level of physical fitness. We propose that the adaptive plasticity is a regulator of dose response; hence it gives shape to the hormesis curve.
Fig. 3.
Exercise with different intensities, using different pathways (anaerobic alactic (AA), anaerobic lactic (AL) and aerobic (AE), to reproduce ATP. Due to the intensity of exercise the causative reason for fatigue and recovery would be dependent on the intensity of exercise. The production of ROS is also dependent on intensity: greater intensity is paired with greater production of ROS. The recovery period to the normalization of ROS production is not well known.
4. Extension of the peak of the hormesis curve with exercise
As we demonstrated earlier, exercise-associated adaptation can extend the functional end-points of adaptation, which means that the distance between the functional/actual endpoints and the biological endpoints (limitations) are significantly narrowed. If we reflect these changes into the bell-shaped hormesis curve it could mean a significant extension of the peak and/or the “optimal” zones (Fig. 4). This extension would mean that a greater dose could be tolerated by the body with high levels of physiological performance.
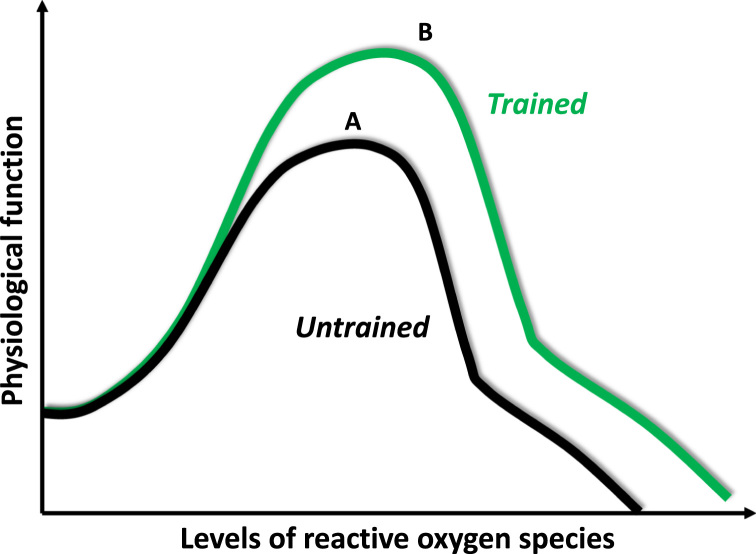
Fig. 4.
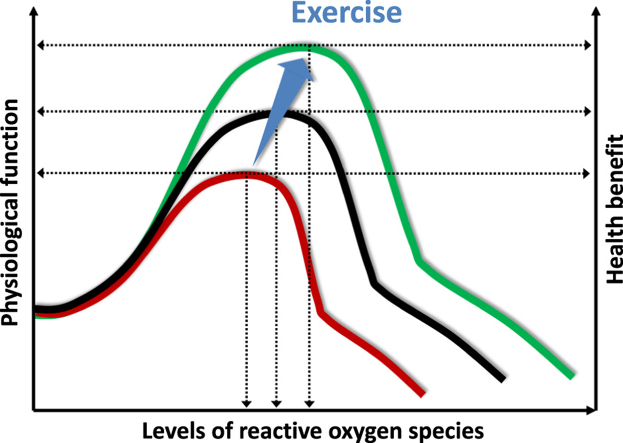
The “A” curve is a typical dose-response curve to physical exercise. Moderate exercise increases the physiological function of different organs, increases the rate of prevention against diseases and improves quality of life. Physical inactivity, strenuous exercise and overtraining increase the risk of diseases and decrease physiological function. The “B” curve indicates that regular exercise can extend or stretch the levels of ROS that are associated with high levels of physical function. This means that exercise can increase tolerance against high levels of ROS and can be preventive against oxidative stress- associated diseases.
Untrained individuals also have a bell-shaped dose response curve to exercise, which means that during moderate levels of intensity and duration their physiological responses would be better. On the other hand, high intensity or exercise of long duration would cause fatigue and decreased performance. The so-called “optimal” intensity or/and duration would comprise a very narrow zone. Superbly trained individuals would endure much larger, and wider “optimal” exercise loading with enhanced functional parameters of exercise. The performance of untrained individual would decrease when his/her lactate reaches 10 mM/L, while superbly trained athletes increase their performance with lactate levels over 20 mM/L. The same situation is true for the dosage of ROS. Well-trained individuals, due to the adaptive response, would endure larger levels of ROS without significant damage to macromolecules, and larger levels of oxidative damage would be tolerated without significant loss of function. This is the effect of exercise-mediated pre-condition via ROS, and is the “dose-response” phenomenon by which exercise attenuates the incidence of ROS associated diseases [22], [27], [29], [45], [57]. We suggest that the so called health promoting effects of exercise are partly due to extension of the summit of bell-shaped dose response curves, representing greater tolerance of ROS levels, without loss of function.
In accordance with the hormesis type of dose response of ROS, recently it has been suggested that this view can be extended to oxidative damage [50]. We use different markers of oxidative damage in order to evaluate the interaction between ROS and macromolecules. Interestingly, the most common oxidative damage markers, such as malondialdehyde (MDA) for lipid peroxidation, carbonyls for oxidative protein damage or 8-oxo-7,8-dihydroguanine (8-oxoG) for DNA damage, are always present in cells. Although the oxidative stress theory is one of the most accepted theories of aging [24], it is interesting that even in the very young organism there is a measurable amount of lipid peroxidation, carbonyl levels or even 8-oxoG. Moreover, the accumulation of MDA, carbonyls or 8-oxoG with aging is not a linear process: the actual increase is in the last quarter of the life span [51]. It appears that large doses of antioxidants cannot eliminate these damage markers, as they have definite physiological effects on individual cells. Indeed, base levels of oxidative modification of lipids can be important for cell signaling, and membrane remodeling. In addition, the ROS-mediated post translation modifications of proteins could be important to the homeostasis of protein turnover [50], while low levels of 8-oxoG might be necessary for transcription [42]. 8-oxoG has been regarded as a damage marker of DNA. However, recent studies try to solve the enigmas, why the guanine with the lowest oxidation potential among DNA bases is present in increased number in the DNA of aerobic organisms compared to anaerobic organisms, and why guanine is present in large concentration in telomeres and in promoter regions of DNA.
It turns out that 8-oxoG, with the complex of its repair enzyme 8-oxoguanine DNA glycosylase1 (OOG1), is involved in cellular signaling [1], [37], [6], and therefore, 8-oxoG-OGG1 complex is more than just a damage/repair agent.
Based on the above mentioned information, we suggest that a moderate level of oxidative damage could be not just a consequence of metabolism, but also even important and necessary for cells. There is no question that high levels of accumulation of lipid peroxidation, oxidative protein damage or 8-oxoG, accelerate the progress of aging and neurodegenerative diseases. Therefore, agents, including moderate levels of oxidative damage, that induce the activity of repair enzymes, such as Ca(2+)-independent phospholipase A (2) (iPLA(2)beta) for lipids, methionine sulfoxidereductase for proteins, and OGG1, are important for the maintenance and viability of cells.
Acute or severe bouts of exercise can lead to moderate increases in oxidative damage, while regular exercise-associated adaptive responses result in increased activity of repair enzymes and moderate levels of oxidative damage [46], [47], [51].
It seems very unlikely that exercise, except that of high intensity and long duration (maximal intensity exercise cannot last long due to the accumulation of fatigue inducing factors like lactic acid, ammonia, iP, AMP etc.), can result in oxidative damage to a degree which could be very harmful for humans.
5. Conclusions
The response of biological systems to stressors can be described by a bell-shaped curve. Physical exercise also evokes this hormesis curve-response by the organism. The two end-points of the hormesis curve are inactivity and overtraining, and both of these result in decreased physiological function. Antioxidant supplementation, depending on the timing, could suppress, enhance or prolong high levels of physiological function, but hardly curb the systemic effects of exercise training. Adaptive capacity could be extended, that is, stretch the top of the bell-shaped curve, resulting in a greater tolerance for ROS, and possibly other metabolic products, with high performance and loss of function. This kind of adaptive response could be important to health promotion and the preventive role of exercise training for certain pathologies and maladies. Oxidative damage markers, such as MDA, carbonyls or 8-oxoG are necessary players for hormesis.
Acknowledgements
This study was supported by OTKA grant (112810) awarded to Z.R. Authors acknowledge the assistance of Professor A.W. Taylor in the preparation of the manuscript. E.K and L.B. were supported by GINOP-2.3.2-15-2016-00062. A.P. was supported by UNKP-UNKP-16-4 New National Excellence Program of the Ministry of Human Capacities.
References
- 1.Aguilera-Aguirre L. Whole transcriptome analysis reveals an 8-oxoguanine DNA glycosylase-1-driven DNA repair-dependent gene expression linked to essential biological processes. Free Radic. Biol. Med. 2015;81:107–118. doi: 10.1016/j.freeradbiomed.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade F.H., Reid M.B., Allen D.G., Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J. Physiol. 1998;509(Pt 2):565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arumugam T.V., Gleichmann M., Tang S.C., Mattson M.P. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res. Rev. 2006;5:165–178. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Bangsbo J. Performance in sports--With specific emphasis on the effect of intensified training. Scand. J. Med. Sci. Sports. 2015;25(Suppl. 4):S88–S99. doi: 10.1111/sms.12605. [DOI] [PubMed] [Google Scholar]
- 5.M.S. Bassil, R. Gougeon, Muscle protein anabolism in type 2 diabetes Current opinion in clinical nutrition and metabolic care, 2013 16:83–88 〈http://dx.doi.org/10.1097/MCO.0b013e32835a88ee〉. [DOI] [PubMed]
- 6.Boldogh I. Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine. J. Biol. Chem. 2012;287:20769–20773. doi: 10.1074/jbc.C112.364620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolli R., Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol. Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 8.Bramble D.M., Lieberman D.E. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 9.Brovold T., Skelton D.A., Bergland A. Older adults recently discharged from the hospital: effect of aerobic interval exercise on health-related quality of life, physical fitness, and physical activity. J. Am. Geriatr. Soc. 2013 doi: 10.1111/jgs.12400. [DOI] [PubMed] [Google Scholar]
- 10.E.J. Calabrese, L.A. Baldwin, U-shaped dose-responses in biology, toxicology, and public health Annual review of public health, 2001 22:15–33 〈http://dx.doi.org/10.1146/annurev.publhealth.22.1.15〉. [DOI] [PubMed]
- 11.Calabrese E.J., Baldwin L.A. Defining hormesis. Hum. Exp. Toxicol. 2002;21:91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- 12.Carter C.S., Hofer T., Seo A.Y., Leeuwenburgh C. Molecular mechanisms of life- and health-span extension: role of calorie restriction and exercise intervention. Appl. Physiol. Nutr. Metab. 2007;32:954–966. doi: 10.1139/H07-085. [DOI] [PubMed] [Google Scholar]
- 13.Clifford T., Bell O., West D.J., Howatson G., Stevenson E.J. Antioxidant-rich beetroot juice does not adversely affect acute neuromuscular adaptation following eccentric exercise. J. Sports Sci. 2017;35:812–819. doi: 10.1080/02640414.2016.1192670. [DOI] [PubMed] [Google Scholar]
- 14.Combes A., Dekerle J., Webborn N., Watt P., Bougault V., Daussin F.N. Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle. Physiol. Rep. 2015:3. doi: 10.14814/phy2.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook R.R., Calabrese E.J. Hormesis is biology, not religion. Environ. Health Perspect. 2006;114:A688. doi: 10.1289/ehp.114-1764167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding D. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388:1311–1324. doi: 10.1016/S0140-6736(16)30383-X. [DOI] [PubMed] [Google Scholar]
- 17.Ding Y.H. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol. 2005;109:237–246. doi: 10.1007/s00401-004-0943-y. [DOI] [PubMed] [Google Scholar]
- 18.Frazzitta G., Balbi P., Maestri R., Bertotti G., Boveri N., Pezzoli G. The beneficial role of intensive exercise on Parkinson disease progression. Am. J. Phys. Med. Rehab. 2013;92:523–532. doi: 10.1097/PHM.0b013e31828cd254. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Cabrera M.C., Borras C., Pallardo F.V., Sastre J., Ji L.L., Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Cabrera M.C. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Cabrera M.C., Salvador-Pascual A., Cabo H., Ferrando B., Vina J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015;86:37–46. doi: 10.1016/j.freeradbiomed.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Hamer M. Psychosocial stress and cardiovascular disease risk: the role of physical activity. Psychosom. Med. 2012;74:896–903. doi: 10.1097/PSY.0b013e31827457f4. [DOI] [PubMed] [Google Scholar]
- 23.Han C. Effects of long-term exercise on age-related hearing loss in mice. J. Neurosci. 2016;36:11308–11319. doi: 10.1523/JNEUROSCI.2493-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 25.Higashida K., Kim S.H., Higuchi M., Holloszy J.O., Han D.H. Normal adaptations to exercise despite protection against oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2011;301:E779–E784. doi: 10.1152/ajpendo.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim C.S., Park S., Chun Y., Song W., Kim H.J., Kim J. Treadmill exercise attenuates retinal oxidative stress in naturally-aged mice: an immunohistochemical study. Int. J. Mol. Sci. 2015;16:21008–21020. doi: 10.3390/ijms160921008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacic J.C., Castellano J.M., Fuster V. Cardiovascular defense challenges at the basic, clinical, and population levels. Ann. N. Y. Acad. Sci. 2012;1254:1–6. doi: 10.1111/j.1749-6632.2012.06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo Y.C., Lin J.C., Bernard J.R., Liao Y.H. Green tea extract supplementation does not hamper endurance-training adaptation but improves antioxidant capacity in sedentary men. Appl. Physiol. Nutr. Metab. 2015;40:990–996. doi: 10.1139/apnm-2014-0538. [DOI] [PubMed] [Google Scholar]
- 29.Lacza G., Radak Z. Is physical activity an elixir. Orv. Hetil. 2013;154:764–768. doi: 10.1556/OH.2013.29616. [DOI] [PubMed] [Google Scholar]
- 30.Loprinzi P.D., Edwards M. Association between objectively measured physical activity and erectile dysfunction among a nationally representative sample of American. Men. J. Sex. Med. 2015;12:1862–1864. doi: 10.1111/jsm.12977. [DOI] [PubMed] [Google Scholar]
- 31.Maslin M.A., Shultz S., Trauth M.H. A synthesis of the theories and concepts of early human evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370:20140064. doi: 10.1098/rstb.2014.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellett L.H., Bousquet G. Cardiology patient page. Heart-healthy exercise. Circulation. 2013;127:e571–e572. doi: 10.1161/CIRCULATIONAHA.112.000880. [DOI] [PubMed] [Google Scholar]
- 33.Michna L. Inhibitory effects of voluntary running wheel exercise on UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis. 2006;27:2108–2115. doi: 10.1093/carcin/bgl057. [DOI] [PubMed] [Google Scholar]
- 34.Mille-Hamard L., Breuneval C., Rousseau A.S., Grimaldi P., Billat V.L. Transcriptional modulation of mitochondria biogenesis pathway at and above critical speed in mice. Mol. Cell Biochem. 2015;405:223–232. doi: 10.1007/s11010-015-2413-3. [DOI] [PubMed] [Google Scholar]
- 35.Morrison D., Hughes J., Della Gatta P.A., Mason S., Lamon S., Russell A.P., Wadley G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015;89:852–862. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 36.Okita K., Kinugawa S., Tsutsui H. Exercise intolerance in chronic heart failure--skeletal muscle dysfunction and potential therapies. Circ. J. 2013;77:293–300. doi: 10.1253/circj.cj-12-1235. [DOI] [PubMed] [Google Scholar]
- 37.Pan L. Oxidized guanine base lesions function in 8-oxoguanine DNA glycosylase-1-mediated epigenetic regulation of nuclear factor kappaB-driven gene expression. J. Biol. Chem. 2016;291:25553–25566. doi: 10.1074/jbc.M116.751453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulsen G. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J. Physiol. 2014;592:1887–1901. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peeri M., Habibian M., Azarbayjani M.A., Hedayati M. Protective effect of aerobic exercise against L-name-induced kidney damage in rats. Arh. Hig. Rada Toksikol. 2013;64:43–49. doi: 10.2478/10004-1254-64-2013-2260. [DOI] [PubMed] [Google Scholar]
- 40.Picklo M.J., Thyfault J.P. Vitamin E and vitamin C do not reduce insulin sensitivity but inhibit mitochondrial protein expression in exercising obese rats. Appl. Physiol. Nutr. Metab. 2015;40:343–352. doi: 10.1139/apnm-2014-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radak Z., Boldogh I. 8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense against oxidative stress. Free Radic. Biol. Med. 2010;49:587–596. doi: 10.1016/j.freeradbiomed.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radak Z., Chung H.Y., Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6:71–75. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- 44.Radak Z., Chung H.Y., Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Radak Z., Hart N., Sarga L., Koltai E., Atalay M., Ohno H., Boldogh I. Exercise plays a preventive role against Alzheimer's disease. J. Alzheimers Dis. 2010;20:777–783. doi: 10.3233/JAD-2010-091531. [DOI] [PubMed] [Google Scholar]
- 46.Radak Z., Ihasz F., Koltai E., Goto S., Taylor A.W., Boldogh I. The redox-associated adaptive response of brain to physical exercise. Free Radic. Res. 2013 doi: 10.3109/10715762.2013.826352. [DOI] [PubMed] [Google Scholar]
- 47.Radak Z. Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radic. Biol. Med. 2013;58:87–97. doi: 10.1016/j.freeradbiomed.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Radak Z., Sasvari M., Nyakas C., Pucsok J., Nakamoto H., Goto S. Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch. Biochem. Biophys. 2000;376:248–251. doi: 10.1006/abbi.2000.1719. [DOI] [PubMed] [Google Scholar]
- 49.Radak Z., Taylor A.W., Ohno H., Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc. Immunol. Rev. 2001;7:90–107. [PubMed] [Google Scholar]
- 50.Radak Z., Zhao Z., Goto S., Koltai E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol. Asp. Med. 2011;32:305–315. doi: 10.1016/j.mam.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Radak Z., Zhao Z., Koltai E., Ohno H., Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013;18:1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reid M.B., Stokic D.S., Koch S.M., Khawli F.A., Leis A.A. N-acetylcysteine inhibits muscle fatigue in humans. J. Clin. Investig. 1994;94:2468–2474. doi: 10.1172/JCI117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ristow M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sato Y., Nagasaki M., Kubota M., Uno T., Nakai N. Clinical aspects of physical exercise for diabetes/metabolic syndrome. Diabetes Res. Clin. Pract. 2007;77(Suppl. 1):S87–S91. doi: 10.1016/j.diabres.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 55.Shill D.D., Southern W.M., Willingham T.B., Lansford K.A., McCully K.K., Jenkins N.T. Mitochondria-specific antioxidant supplementation does not influence endurance exercise training-induced adaptations in circulating angiogenic cells, skeletal muscle oxidative capacity or maximal oxygen uptake. J. Physiol. 2016;594:7005–7014. doi: 10.1113/JP272491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strasser B. Physical activity in obesity and metabolic syndrome. Ann. New Y. Acad. Sci. 2013;1281:141–159. doi: 10.1111/j.1749-6632.2012.06785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sturek M. Ca2+ regulatory mechanisms of exercise protection against coronary artery disease in metabolic syndrome and diabetes. J. Appl. Physiol. (Bethesda, Md: 1985) 2011;111:573–586. doi: 10.1152/japplphysiol.00373.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Theodorou A.A. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am. J. Clin. Nutr. 2011;93:1373–1383. doi: 10.3945/ajcn.110.009266. [DOI] [PubMed] [Google Scholar]
- 59.Torma F. Exercise increases markers of spermatogenesis in rats selectively bred for low running capacity. PLoS One. 2014;9:e114075. doi: 10.1371/journal.pone.0114075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Huffel L., Tomson C.R., Ruige J., Nistor I., Van Biesen W., Bolignano D. Dietary restriction and exercise for diabetic patients with chronic kidney disease: a systematic review. PLoS One. 2014;9:e113667. doi: 10.1371/journal.pone.0113667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venditti P., Napolitano G., Barone D., Di Meo S. Vitamin E supplementation modifies adaptive responses to training in rat skeletal muscle. Free Radic. Res. 2014;48:1179–1189. doi: 10.3109/10715762.2014.937341. [DOI] [PubMed] [Google Scholar]
- 62.Vining A.Q., Nunn C.L. Evolutionary change in physiological phenotypes along the human lineage. Evol. Med. Public Health. 2016;2016:312–324. doi: 10.1093/emph/eow026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan Z., Okutsu M., Akhtar Y.N., Lira V.A. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 2011;110:264–274. doi: 10.1152/japplphysiol.00993.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yfanti C. Antioxidant supplementation does not alter endurance training adaptation. Med. Sci. Sports Exerc. 2010;42:1388–1395. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]
- 65.Yilmaz Y., Colak Y., Kurt R., Senates E., Eren F. Linking nonalcoholic fatty liver disease to hepatocellular carcinoma: from bedside to bench and back. Tumori. 2013;99:10–16. doi: 10.1177/030089161309900102. [DOI] [PubMed] [Google Scholar]
- 66.Zhu L., Ye T., Tang Q., Wang Y., Wu X., Li H., Jiang Y. Exercise preconditioning regulates the toll-like receptor 4/nuclear factor-kappab signaling pathway and reduces cerebral ischemia/reperfusion inflammatory injury: a study in rats. J. Stroke Cereb. Dis. 2016;25:2770–2779. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.033. [DOI] [PubMed] [Google Scholar]