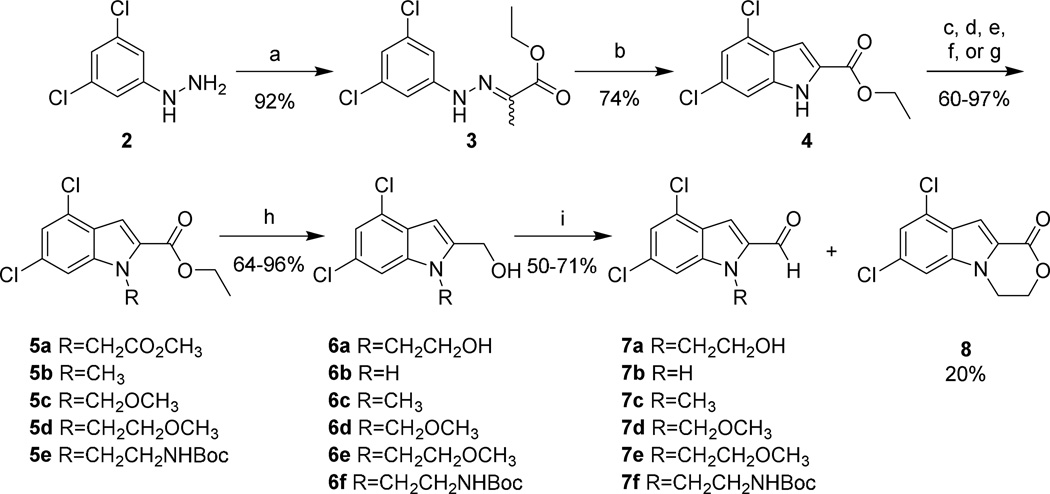
Scheme 1. Synthesis of the 3,5-dichloroindole western end of 1 and its analogs.
Reagents and conditions: (a) ethyl pyruvate, EtOH, reflux, 1 hr; (b) PPA, 130 °C, 10 min; (c) methyl bromoacetate, K2CO3, DMF, rt, o.n.; (d) NaH, DMF, 0 °C, 30 min; then CH3I, 0 °C→rt, 3 hrs; (e) NaH, DMF, 0 °C, 30 min; then CH3OCH3Cl , 0 °C→rt, 3 hrs; (f) NaH, DMF, 0 °C, 30 min; then CH3OCH2CH2Br , 0 °C→rt, 18 hrs; (g) K2CO3, DMF, BocNH(CH2)2OTs, rt, o.n.; (h) DIBAL, THF, rt, 1 hr; (i) MnO2, THF, rt, 2 hrs.