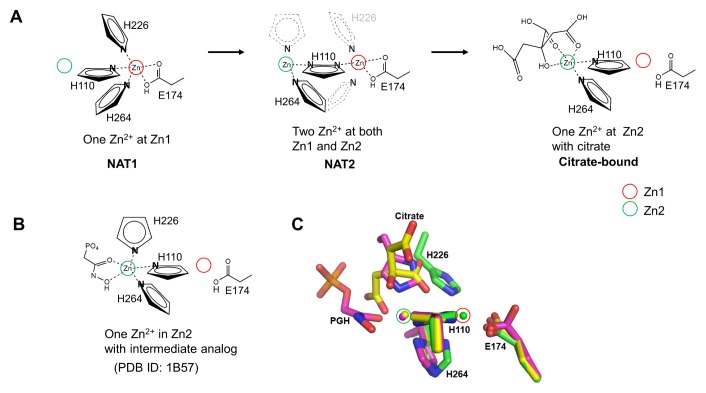
Fig. 3.
Metal-binding site structures of EcFBA. (A) Schematic drawing of metal shift in the EcFBA active site with corresponding conformational changes of coordinating residues. Only coordinating nitrogen atom in histidine residues is shown. In NAT2 structure, flip-flop dual conformations of His110 are shown as two nitrogen atoms in a single imidazole ring. His226 residue is not shown due to disordered conformations, and the proposed positions are represented with grey dashed lines. His264 residue is shown as dual conformations to coordinate Zn2+ at Zn1 or Zn2. (B) Schematic drawing of phosphoglycolohydroxamate (PGH)-bound EcFBA structure (2). (C) Comparison between native and ligand-bound EcFBA structures. Superimposed active site structures between NAT1 (green), citrate-bound (yellow), and phosphoglycolohydroxamate (PGH)-bound (purple) EcFBA structures.