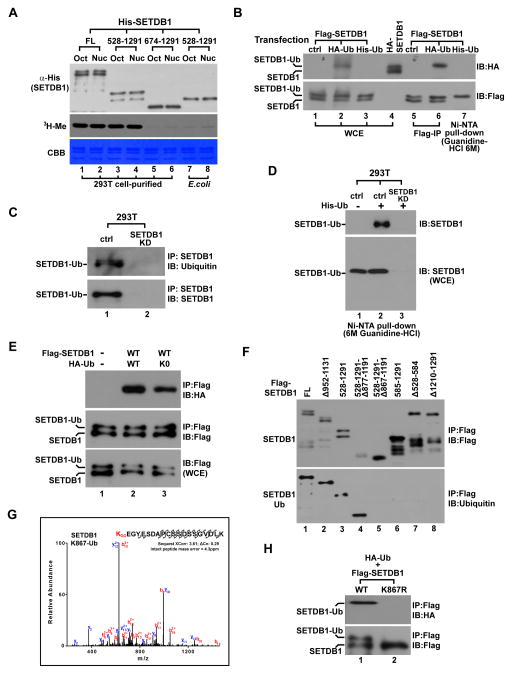
Figure 1. SETDB1 is monoubiquitinated at K867.
(A) Western blot (top), autoradiogram (middle) and gel image (bottom) of in vitro histone methylation (HMT) assay. His-tagged SETDB1-FL or different indicated deletions were purified from transfected 293T cells or bacteria using Ni-NTA agarose and was incubated with histone octamer (Oct) or mononucleosome (Nuc) for reaction.
(B) Immunoblot (IB) of Flag- or HA-tagged SETDB1 after it was co-expressed with HA- or His-tagged Ub in 293T cells. Flag-SETDB1 was IPed using Flag antibody while His-SETDB1 was purified by Ni-NTA pull-down under the denaturing condition. WCE represents whole cell extract.
(C) Endogenous SETDB1 was IP-purified from control and SETDB1 stable knockdown (SETDB1-KD) 293T cells followed by immunoblot analysis using indicated antibodies.
(D) His-Ub was expressed in control and SETDB1-KD 293T cells followed by Ni-NTA pull-down under the denaturing condition. WCE and purified proteins were next analyzed by immunoblot (IB) using SETDB1 antibody.
(E) Flag-SETDB1 was co-expressed with HA- Ub-wt or Ub-K0 in 293T cells followed by IP-western analysis using indicated antibodies.
(F) Flag-tagged SETDB1- FL or different deletions were IPed from transfected cells and analyzed by western blot using Flag or Ub antibody.
(G) LC-Mass Spectrometry analysis of SETDB1 ubiquitination. After Flag-SETDB1-FL was expressed in 293T cells, IPed and resolved by SDS-PAGE, the top ubiquitinated protein band was excised for LC-Mass. Arrows are the fragment ions that confirm K867 as the ubiquitination site.
(H) Flag-SETDB1-wt or K867R was co-expressed with HA-Ub in 293T cells followed by IP-western analysis.
See also Figure S1.