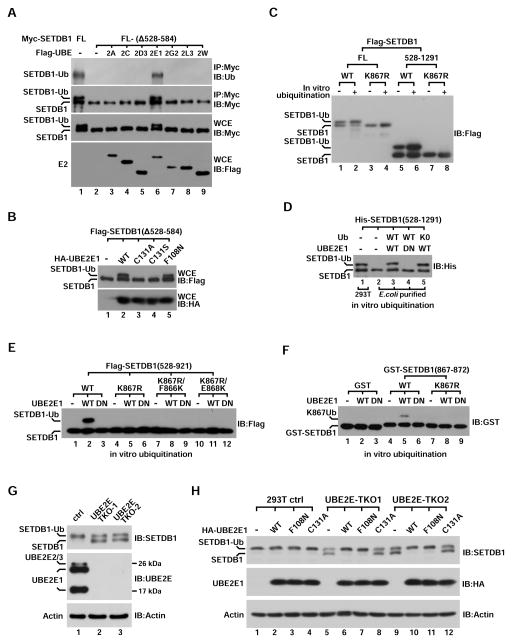
Figure 2. UBE2E enzymes catalyze SETDB1-K867Ub1 independent of E3.
(A) Myc-tagged SETDB1- FL or (Δ528–584) deletion was co-expressed with different Flag-tagged E2s in 293T cells followed by IP-western analysis using indicated antibodies. Protein expression was indicated by western blot of whole cell extract (WCE).
(B) Western blot analysis of WCE derived from 293T cells co-expressing Flag-SETDB1(Δ528–584) and UBE2E1-wt or its different mutants.
(C) Flag-tagged SETDB1- FL or (528–1291) (wt or K867R) was IP-purified from transfected cells and then incubated with recombinant UBE2E1 for in vitro ubiquitination. Products were analyzed by western blot using Flag antibody.
(D) Immunoblot of bacteria-purified His-SETDB1(528–1291) after it was in vitro ubiquitinated with Ub-wt or Ub-K0 by recombinant UBE2E1-wt or C131A (DN).
(E) IP-purified Flag-SETDB1(528–921)-wt or three indicated mutants were ubiquitinated in vitro by recombinant UBE2E1-wt or C131A (DN) and analyzed by anti-Flag western blot.
(F) Anti-GST western blot analysis of purified GST-SETDB1(867–872) fusion protein (wt or K867R) after it was in vitro ubiquitinated by UBE2E1-wt or C131A (DN).
(G) WCEs derived from control and two UBE2E triple-knockout (TKO) 293T cells were analyzed by immunoblot with indicated antibodies. TKO1 and TKO2 cells were derived from different UBE2E2/3 sgRNAs.
(H) Control and two UBE2E-TKO 293T cells were rescued by transient expression of HA-UBE2E1- wt or different mutant. WCEs were then analyzed by immunoblots using indicated antibodies.
See also Figure S2.