Figure 3. Random forest analysis of miR data using the sequencing approach.
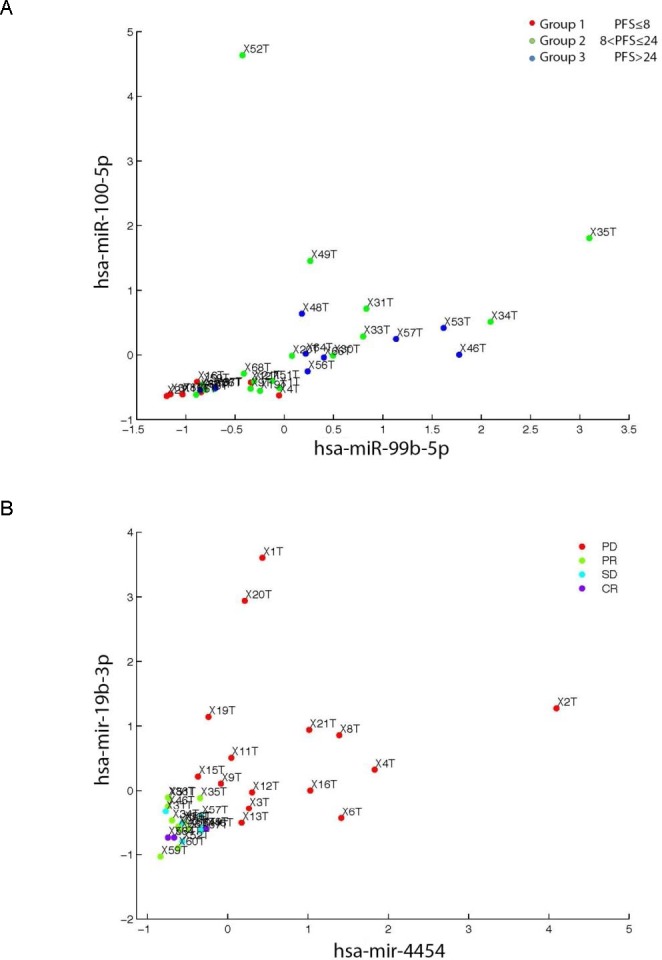
A: The two top candidate miRs (hsa-miR-100-5p, hsa-miR99b-5p) associating with PFS were evaluated. Group 1 (PFS≤8 months; n=8) Group 2 (8<PFS≤24; n=18) and 24<PFS (n=9) as a group 3. E.g.: X1T indicate patient 1 tumor sample and analogously for the other patients labels. Combination of the two top miRs selected based on the random forest analysis showed grouping of patients from group 1 (PFS<8 months) from group 2 and 3 (PFS ≥ 8 months). Such a marker/markers may potentially improve selection of the ccRCC patients for the TKI treatment. B: two top candidate miRs (hsa-miR-19b-3p, hsa-miR-4454) evaluated using the RECIST labeling. PD patients presented as red dot, SD as blue dot, PR as green dot and CR as violet dot. E.g.: X1T indicate patient 1 tumor sample and analogously for the other patients labels. Presented top targets successfully showed distinct grouping of PD patients after TKI treatment was applied. ccRCC - clear cell renal cell carcinoma, PFS - progression free survival, PD- progressive disease, SD- stable disease, PR- partial response, CR- complete response, TKI - tyrosine kinase inhibitor.
