Figure 6. Inhibition of NF-κB transcriptional activity by selinexor is associated with nuclear localization of IκB-α and protection from degradation.
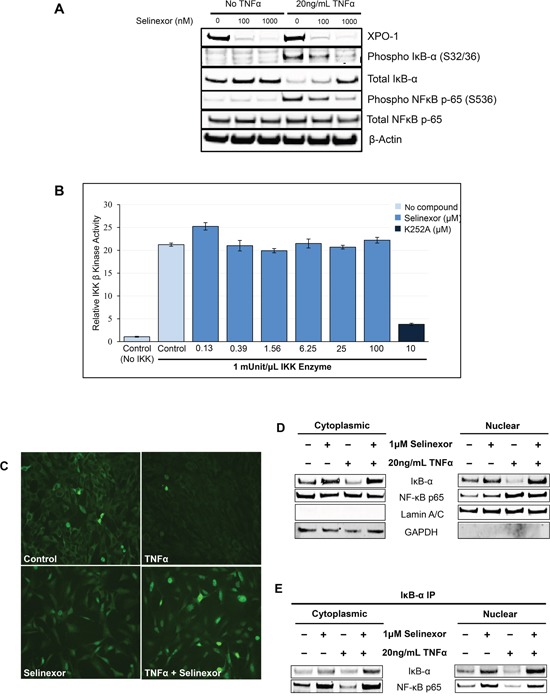
A. U-2 OS cells were stimulated with or without 20ng/mL TNFα for 2 hours before being treated with vehicle, 100nM or 1μM selinexor for the next 24 hours. Western blot of phospho-IκB-α and phosphor-NF-κB p-65 shows that selinexor reverts the pro-inflammatory effects of TNFα and selinexor also increased cellular levels of IκB-α. Selinexor induces XPO1 degradation. It is mediated by the proteasome degradation pathway (TK and YL, not shown). B. IKKβ kinase activity was analyzed by in vitro kinase assay using recombinant IKKβ, recombinant IκB-α substrate containing serine 32/36 residues and selinexor at different concentrations. IKKβ kinase activity was detected using a phosphorylation specific IκB-α antibody. Selinexor had no inhibitory effects on IKKβ kinase activity. The pan-kinase inhibitor K252A was used as a positive control for the assay. C. Immunofluorescence staining of IκB-α after treatment with 20ng/mL TNFα or/and 1μM selinexor for 24 hours. Selinexor induced nuclear localization of IκB-α in the presence or absence of TNFα. D. Cellular fractionation of U-2 OS cells shows similar increased nuclear levels of IκB-α and NF-κB p65 upon selinexor treatment even in the presence of TNFα. Lamin A/C was used as nuclear protein marker; GAPDH as a cytosolic protein marker. E. IκB-α immunoprecipitation (IP) and Western blotting of cytoplasmic and nuclear fractions of U-2OS cells treated with selinexor and TNFα shows that IκB-α binds to NF-κB p65 subunit both in the nucleus and cytoplasm.
