Figure 6. Up- or down-regulation of miR-31 in CAFs regulated the radiosensitivity and X-ray-induced apoptosis of co-cultured CRC cells.
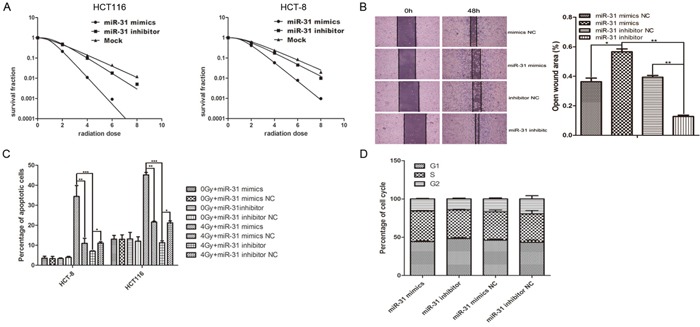
A. (Left) HCT116 cells were exposed to X-rays at different irradiation doses, 0, 2, 4, 6, and 8 Gy, after co-culture with CAFs that were transfected with miR-31 mimics, miR-31 inhibitor or mock (negative control) for 48 h. After 14 days, the surviving fraction was calculated as a ratio of the number of colonies formed divided by the total number of cells plated times the plating efficiency. (Right) the surviving fraction of HCT-8 In all, up- and down-regulation of miR-31 in CAFs affected the radiation sensitivity of colorectal cancer cells. B. Wound healing assay of HCT-8 cells co-cultured with CAF regulated miR-31. Wound healing was observed 48 h after the treatment, and the open wound area was normalized to the area at the initial time that the wound was made. The data are presented as the means ± SEM and normalized to the control cells (*P < 0.05, **P < 0.01) C. Flow cytometry assays were used to determine the amount of apoptosis that occurred in HCT116 and HCT-8 cells that were co-cultured with CAFs transfected with miR-31 mimic, a miR-31 mimic negative control, miR-31 inhibitor or a miR-31 inhibitor negative control. The percentage of apoptotic cells was determined in the non-transfected and transfected cells were that subjected to 0 and 8-Gy irradiation. The data are shown as the mean ± SEM of three independent experiments. The miR-31 mimic significantly increased X-ray-induced apoptosis in both cell lines compared with the miR-31 mimic negative control (**P < 0.01) and the miR-31 inhibitor (***P < 0.001), and the miR-31 inhibitor decreased X-ray-induced apoptosis in both cell lines compared with the miR-31 inhibitor negative control (*P < 0.05). D. miR-31 regulated in CAF had no effect on cell cycle progression of colorectal cancer cells HCT-8. Cell cycle analyses were done on co-cultured HCT-8, cells transduced with PI in preparation for flow cytometry with the FACScan/Cell FIT system as described in Materials and methods. Wound healing assay of HCT-8 cells co-cultured with CAF regulated miR-31. Wound healing was observed 48 h after the treatment, and the open wound area was normalized to the area at the initial time that the wound was made. The data are presented as the means ± SEM and normalized to the control cells.
