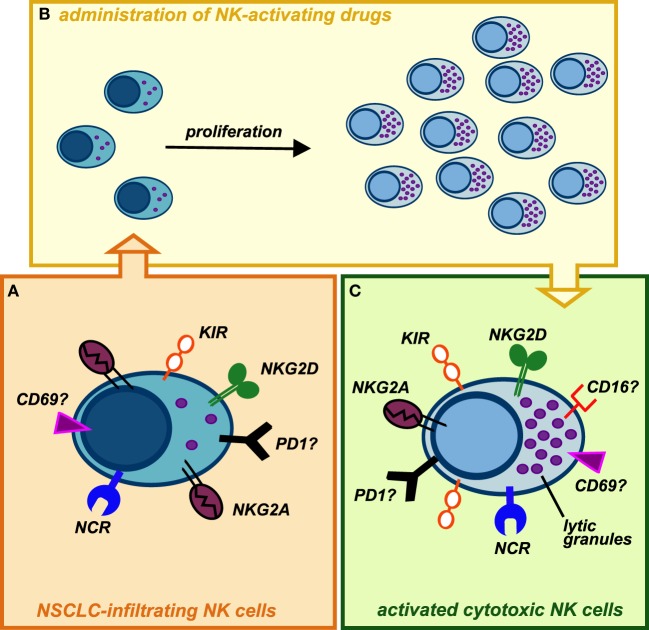
Figure 2.
Possible conversion of lung cancer-infiltrating natural killer (NK) cells into highly cytotoxic killer cells upon administration of NK-activating drugs. (A) NK cells isolated from NSCLC tissues display phenotypic features similar to those of peripheral blood (PB) CD56bright NK cells, with the exception of KIRs, that can be detectable on these cells. Similarly to PB CD56bright NK cells, they are endowed with high proliferative potential but impaired in their cytotoxic capability. In addition, it is not clear whether NK cells infiltrating human lung tumors express other immune checkpoints (such as PD-1), which might further contribute to their poor cytolytic activity. (B) Upon activation with stimulating cytokines (recombinant IL-15, IL-2) or mAbs-blocking inhibitory receptors (e.g., anti-NKG2A, which is highly expressed on these cells, or anti-PD1), intratumoral NK cells may proliferate in situ and modify their phenotype acquiring strong cytolytic capabilities against cancer cells. (C) Administration of cytokines or other NK-activating drugs, currently available, can upregulate the expression of cytolytic granules on expanded tumor-infiltrating NK cells but also induce higher levels of KIR inhibitory receptors: this might be circumvented by administration of anti-KIRs mAbs. It remains to be investigated whether in vivo stimulation of lung cancer-infiltrating NK cells can upregulate on these cells the expression of activating receptors (e.g., CD16), immune checkpoints, or markers of tissue residency.