The reaction of silver nitrate with the ligands 2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine, 2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine and 2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine, three tetrakis-thioether-substituted pyrazine ligands, lead, respectively, to the formation of compounds with a metal–organic chain, a metal–organic network and a metal–organic framework structure.
Keywords: crystal structure, tetrakis-thioether-substituted pyrazines, silver(I) nitrate, metal–organic chain (MOC), metal–organic network (MON), metal–organic framework (MOF), C—H⋯O and C—H⋯S hydrogen bonds
Abstract
The reaction of the ligand 2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine (L1) with silver(I) nitrate led to {[Ag(C12H20N2S4)](NO3)}n, (I), catena-poly[[silver(I)-μ-2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine] nitrate], a compound with a metal–organic chain structure. The asymmetric unit is composed of two half ligands, located about inversion centres, with one ligand coordinating to the silver atoms in a bis-tridentate manner and the other in a bis-bidentate manner. The charge on the metal atom is compensated for by a free nitrate anion. Hence, the silver atom has a fivefold S3N2 coordination sphere. The reaction of the ligand 2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine (L2) with silver(I) nitrate, led to [Ag2(NO3)2(C32H28N2S4)]n, (II), poly[di-μ-nitrato-bis{μ-2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine}disilver], a compound with a metal–organic network structure. The asymmetric unit is composed of half a ligand, located about an inversion centre, that coordinates to the silver atoms in a bis-tridentate manner. The nitrate anion coordinates to the silver atom in a bidentate/monodentate manner, bridging the silver atoms, which therefore have a sixfold S2NO3 coordination sphere. The reaction of the ligand 2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine (L3) with silver(I) nitrate led to [Ag3(NO3)3(C28H24N6S4)]n, (III), poly[trinitrato{μ 6-2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine}trisilver(I)], a compound with a metal–organic framework structure. The asymmetric unit is composed of half a ligand, located about an inversion centre, that coordinates to the silver atoms in a bis-tridentate manner. One pyridine N atom bridges the monomeric units, so forming a chain structure. Two nitrate O atoms also coordinate to this silver atom, hence it has a sixfold S2N2O2 coordination sphere. The chains are linked via a second silver atom, located on a twofold rotation axis, coordinated by the second pyridine N atom. A second nitrate anion, also lying about the twofold rotation axis, coordinates to this silver atom via an Ag—O bond, hence this second silver atom has a threefold N2O coordination sphere. In the crystal of (I), the nitrate anion plays an essential role in forming C—H⋯O hydrogen bonds that link the metal–organic chains to form a three-dimensional supramolecular structure. In the crystal of (II), the metal–organic networks (lying parallel to the bc plane) stack up the a-axis direction but there are no significant intermolecular interactions present between the layers. In the crystal of (III), there are a number of C—H⋯O hydrogen bonds present within the metal–organic framework. The role of the nitrate anion in the formation of the coordination polymers is also examined.
Chemical context
A series of tetrakis-thioether pyrazine ligands have been prepared in order to study their coordination behaviour with various transition metals (Assoumatine, 1999 ▸). The ligands 2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine (L1), 2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine (L2) and 2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine (L3), were synthesized by the reaction of 2,3,5,6-tetrakis(bromomethyl)pyrazine (Assoumatine & Stoeckli-Evans, 2014b
▸), with the appropriate 2-mercapto derivative. Their crystal structures and syntheses have been reported previously: L1 (Assoumatine & Stoeckli-Evans, 2014a
▸), L2 (Assoumatine et al., 2007 ▸) and L3 (Assoumatine & Stoeckli-Evans, 2016 ▸). The reaction of similar ligands with various silver(I) salts have also resulted in the formation of coordination polymers. For example, 2-{[(pyridin-4-ylmethyl)sulfanyl]methyl}pyrazine (Black & Hanton, 2007 ▸) led to metal–organic frameworks, while ligands 2,3-bis{[(pyridin-2-ylmethyl)sulfanyl]methyl}pyrazine (Caradoc-Davies & Hanton, 2001 ▸) and 2,5-bis{[(pyridin-2-ylmethyl)sulfanyl]methyl}pyrazine (Caradoc-Davies et al., 2001 ▸) both resulted in compounds with metal–organic chains.


Structural commentary
The reaction of the ligand 2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine (L1) with silver(I) nitrate, led to the formation of a metal–organic chain (MOC) structure, (I) (Fig. 1 ▸). Selected bond lengths and angles involving the Ag1 atom are given in Table 1 ▸. The asymmetric unit is composed of two half ligands, located about inversion centres, with one ligand coordinating to the silver atom in a bis-tridentate manner and the other in a bis-bidentate manner. Their pyrazine rings are almost normal to one another, making a dihedral angle of 88.6 (2)°. The charge on the metal atom is compensated for by a free nitrate anion. The silver atom, Ag1, has a fivefold S3N2 coordination sphere with a highly distorted shape and a τ 5 value of 0.63 (τ 5 = 0 for an ideal square-pyramidal coordination sphere, and = 1 for an ideal trigonal-pyramidal coordination sphere; Addison et al., 1984 ▸). Within the MOC structure, there are significant C—H⋯S interactions present, involving the thioether substituent that does not coordinate to the silver atom, viz. atom S3 (Table 4 and Fig. 1 ▸).
Figure 1.
The molecular entities of compound (I), with atom labelling for the asymmetric unit. Unlabelled atoms are related to labelled atoms by symmetry operation (i) = −x, −y + 1, −z + 1, for the ligand involving atom N2, and by symmetry operation (ii) = −x + 1, −y + 1, −z + 2, for the ligand involving atom N1. Displacement ellipsoids are drawn at the 50% probability level. The intramolecular C—H⋯S contacts are shown as dashed lines (see Table 4 ▸).
Table 1. Selected geometric parameters (Å, °) for (I) .
| Ag1—N1 | 2.714 (4) | Ag1—S2 | 2.5987 (16) |
| Ag1—N2 | 2.436 (5) | Ag1—S4i | 2.5910 (15) |
| Ag1—S1 | 2.5895 (15) | ||
| N1—Ag1—N2 | 167.75 (13) | N2—Ag1—S2 | 109.60 (11) |
| N1—Ag1—S1 | 64.36 (9) | N2—Ag1—S4i | 77.43 (10) |
| N1—Ag1—S2 | 72.54 (9) | S1—Ag1—S2 | 129.99 (5) |
| N1—Ag1—S4i | 113.79 (9) | S1—Ag1—S4i | 111.41 (5) |
| N2—Ag1—S1 | 107.74 (11) | S4i—Ag1—S2 | 108.26 (5) |
Symmetry code: (i)  .
.
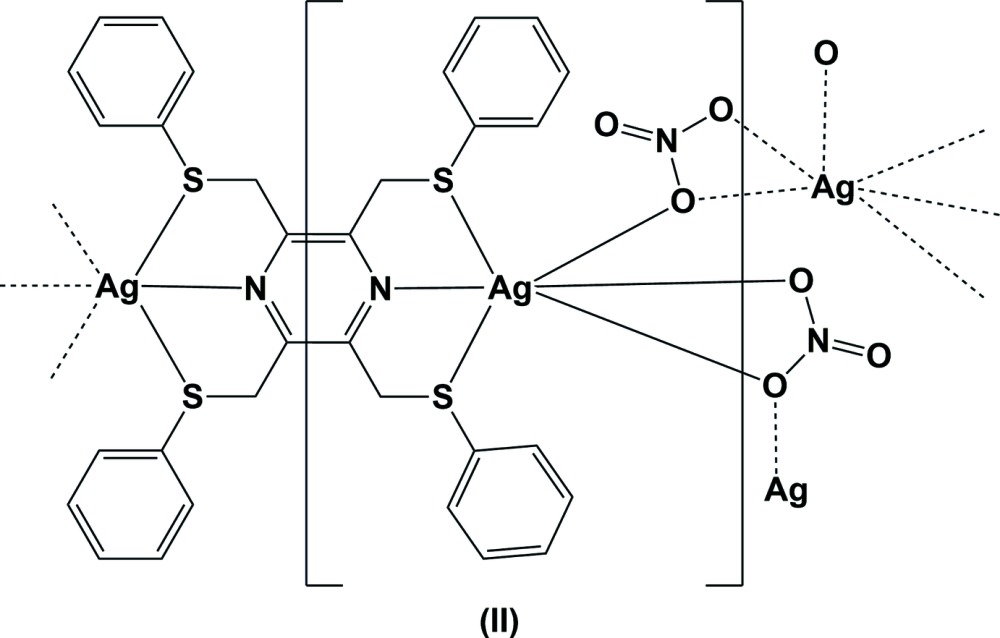
The reaction of the ligand 2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine (L2) with silver(I) nitrate, led to the formation of a metal–organic network (MON) structure, (II) (Fig. 2 ▸). Selected bond lengths and angles involving atom Ag1 are given in Table 2 ▸. The asymmetric unit is composed of half a ligand, located about an inversion centre, a silver atom and a nitrate anion. The ligand coordinates to the silver atoms in a bis-tridentate manner. The nitrate anion coordinates to the silver atom in a bidentate/monodentate manner, bridging the silver atoms, which therefore have a sixfold S2NO3 coordination sphere, best described as a highly distorted octahedron (Table 2 ▸).
Figure 2.
The molecular entities of compound (II), with atom labelling for the asymmetric unit. For the ligand, unlabelled atoms are related to the labelled atoms by symmetry operation (i) −x + 2, −y + 2, −z + 1; other symmetry codes are (ii) x, −y +  , z +
, z +  ; (iii) −x + 2, y +
; (iii) −x + 2, y +  , −z +
, −z +  . Displacement ellipsoids are drawn at the 50% probability level.
. Displacement ellipsoids are drawn at the 50% probability level.
Table 2. Selected geometric parameters (Å, °) for (II) .
| Ag1—N1 | 2.527 (4) | Ag1—O1 | 2.551 (4) |
| Ag1—S1 | 2.6560 (15) | Ag1—O2 | 2.507 (4) |
| Ag1—S2i | 2.6790 (14) | Ag1—O2ii | 2.539 (4) |
| N1—Ag1—S1 | 76.40 (9) | O2ii—Ag1—O1 | 49.56 (12) |
| N1—Ag1—S2i | 70.89 (9) | O2—Ag1—S1 | 80.10 (11) |
| S1—Ag1—S2i | 146.98 (4) | O2ii—Ag1—S1 | 101.67 (11) |
| O2—Ag1—N1 | 112.54 (12) | O1—Ag1—S1 | 120.09 (11) |
| O2—Ag1—O2ii | 117.32 (8) | O2—Ag1—S2i | 116.46 (10) |
| N1—Ag1—O2ii | 128.98 (12) | O2ii—Ag1—S2i | 95.47 (11) |
| O2—Ag1—O1 | 75.15 (13) | O1—Ag1—S2i | 92.47 (11) |
| N1—Ag1—O1 | 163.34 (14) |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
The reaction of the ligand 2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine (L3) with silver(I) nitrate, led to the formation of a metal–organic framework (MOF) structure, (III) (Fig. 3 ▸). Selected bond lengths and angles involving atoms Ag1 and Ag2 are given in Table 3 ▸. The asymmetric unit is composed of half a ligand, located about an inversion centre, a silver atom and a nitrate anion, plus half a second AgNO3 unit located about a twofold rotation axis. The organic ligand coordinates to the silver atoms (Ag1), in a bis-tridentate manner. One pyridine N atom, N2, bridges the monomeric units, so forming a chain structure along the b-axis direction. The nitrate O atoms, O11 and O13, coordinate to silver atom Ag1, hence it has a highly distorted octahedral S2N2O2 coordination sphere (Table 3 ▸). The chains are linked via a second silver atom, Ag2, located on a twofold rotation axis, coordinated by the second pyridine N atom, N3. A second nitrate anion, also lying about the twofold rotation axis, coordinates to this silver atom via an Ag2—O21 bond, hence silver atom Ag2 has a T-shaped N2O coordination sphere.
Figure 3.
The molecular entities of compound (III), with atom labelling for the asymmetric unit. For the ligand, unlabelled atoms are related to the labelled atoms by symmetry operation (ii) −x +  , −y +
, −y +  , −z; other symmetry codes are (i) −x, −y + 1, −z; (iii) −x + 1, y, −z +
, −z; other symmetry codes are (i) −x, −y + 1, −z; (iii) −x + 1, y, −z +  ; (iv) x +
; (iv) x +  , y −
, y −  , z. Displacement ellipsoids are drawn at the 50% probability level.
, z. Displacement ellipsoids are drawn at the 50% probability level.
Table 3. Selected geometric parameters (Å, °) for (III) .
| Ag1—N1 | 2.578 (3) | Ag1—O11 | 2.700 (5) |
| Ag1—N2i | 2.267 (3) | Ag1—O13 | 2.752 (5) |
| Ag1—S1 | 2.7943 (13) | Ag2—N3 | 2.208 (3) |
| Ag1—S2ii | 2.6010 (11) | Ag2—O21 | 2.567 (5) |
| N1—Ag1—N2i | 155.31 (11) | S2ii—Ag1—O13 | 120.26 (10) |
| S1—Ag1—S2ii | 122.71 (3) | O11—Ag1—N1 | 73.76 (11) |
| S1—Ag1—N1 | 68.98 (7) | O11—Ag1—N2i | 99.33 (12) |
| S1—Ag1—N2i | 96.92 (8) | O13—Ag1—N1 | 69.73 (11) |
| S2ii—Ag1—N1 | 70.29 (7) | O13—Ag1—N2i | 88.28 (12) |
| S2ii—Ag1—N2i | 133.03 (8) | O11—Ag1—O13 | 45.99 (14) |
| S1—Ag1—O11 | 122.18 (10) | N3—Ag2—N3iii | 175.41 (12) |
| S1—Ag1—O13 | 79.78 (10) | O21—Ag2—N3 | 92.30 (9) |
| S2ii—Ag1—O11 | 81.18 (10) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
It can be seen from Tables 1 ▸–3 ▸ ▸ that the Ag—N(pyrazine) and Ag—S bond lengths differ considerably for the three compounds. In compound (I), the Ag1—N2 bond length, involving the ligand that coordinates in a bis-bidentate manner, is considerably shorter at 2.436 (5) Å, compared to the Ag1—N1 bond length of 2.714 (4) Å, involving the ligand that coordinates in a bis-tridentate manner. These Ag—N(pyrazine) bond lengths contrast with those for compounds (II) and (III), where both ligands coordinate in a bis-tridentate manner, with values of 2.527 (4) and 2.578 (3) Å, respectively. The Ag1—S bond lengths in compound (I) are almost the same, varying from 2.5895 (15) to 2.5987 (16) Å. These distances are shorter than those in (II), which are 2.6560 (15) and 2.6790 (14) Å, but similar to bond length Ag1—S2ii = 2.6010 (11) Å in (III). The longest Ag—S distance [2.7943 (13) Å] is found for bond Ag1—S1 in (III). Finally, in compound (III), the two Ag—N(pyridine) bond lengths also differ; Ag1—N2i is 2.267 (3) Å, while bond length Ag2—N3 is shorter at 2.208 (3) Å (see Table 3 ▸). Despite the large variation in the Ag—N(pyrazine), Ag—S or Ag—N(pyridine) bond lengths, which perhaps indicates how flexible the ligands are, the values are within the limits observed for similar silver coordinating pyrazine, thioether or pyridine ligands, when compared to the values observed for such structures present in the Cambridge Structural Database (Groom et al., 2016 ▸). The various histograms of the bond lengths have skewed-right distributions and the values vary from 2.10 to 2.75 Å for Ag—N(pyrazine), from 2.48 to 2.79 Å for Ag—S, and 1.90 to 2.99 Å for Ag—N(pyridine).
Supramolecular features
In the crystal of (I), the metal–organic chains (Fig. 4 ▸) propagate along [101]. They are linked via a number of C—H⋯O hydrogen bonds (Table 4 ▸), forming a three-dimensional supramolecular structure, as illustrated in Fig. 5 ▸.
Figure 4.
A partial view, normal to plane (1 0), of the metal–organic chain structure of compound (I). The H atoms have been omitted for clarity
0), of the metal–organic chain structure of compound (I). The H atoms have been omitted for clarity
Table 4. Hydrogen-bond geometry (Å, °) for (I) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C9—H9C⋯S2 | 0.96 | 2.86 | 3.650 (8) | 141 |
| C11—H11A⋯S3 | 0.97 | 2.74 | 3.502 (6) | 136 |
| C11—H11B⋯O13A | 0.97 | 2.52 | 3.438 (17) | 157 |
| C2—H2A⋯O11ii | 0.97 | 2.55 | 3.460 (9) | 156 |
| C2—H2B⋯O12A iii | 0.97 | 2.53 | 3.431 (15) | 154 |
| C3—H3C⋯O12A iii | 0.96 | 2.37 | 3.171 (17) | 141 |
| C3—H3C⋯O12B iii | 0.96 | 2.57 | 3.364 (16) | 140 |
| C6—H6A⋯O13A i | 0.96 | 2.52 | 3.375 (19) | 149 |
| C9—H9A⋯O11iv | 0.96 | 2.58 | 3.503 (10) | 162 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Figure 5.
A view along the b axis of compound (I), with emphasis on the crystal packing. Hydrogen bonds are shown as dashed lines (see Table 4 ▸), and only those H atoms involved in intermolecular C—H⋯O hydrogen bonds have been included.
In the crystal of (II), the metal–organic networks extend parallel to the bc plane and stack up the a axis (Fig. 6 ▸), but there are no significant intermolecular interactions present between the layers.
Figure 6.
A view along the a axis of compound (II), illustrating the role of the NO3 − anion in forming the network structure. H atoms have been omitted for clarity
In the crystal of (III), the metal–organic framework (Fig. 7 ▸) is reinforced by a number of C—H⋯O hydrogen bonds (Table 5 ▸). The voids in this three-dimensional structure, occupied by disordered solvent molecules, amount to only ca 3.7% of the total volume of the unit cell.
Figure 7.
A view along the c axis of compound (III). H atoms have been omitted for clarity
Table 5. Hydrogen-bond geometry (Å, °) for (III) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C11—H11⋯O21 | 0.94 | 2.57 | 3.287 (5) | 133 |
| C3—H3B⋯O21iv | 0.98 | 2.40 | 3.253 (4) | 145 |
| C3—H3B⋯O22iv | 0.98 | 2.49 | 3.420 (6) | 158 |
| C7—H7⋯O13v | 0.94 | 2.51 | 3.268 (6) | 138 |
| C9—H9A⋯O22iv | 0.98 | 2.32 | 3.291 (6) | 171 |
| C12—H12⋯O11vi | 0.94 | 2.51 | 3.310 (7) | 142 |
| C14—H14⋯O22iv | 0.94 | 2.59 | 3.349 (7) | 138 |
Symmetry codes: (iv)  ; (v)
; (v)  ; (vi)
; (vi)  .
.
Database survey
A search of the Cambridge Structural Database (Version 5.38, first update November 2016; Groom et al., 2016 ▸) for tetrakis-substituted pyrazine ligands gave 774 hits, which include 194 hits for compounds involving tetramethylpyrazine. The first such ligand, tetrakis-2,3,5,6-(2′-pyridyl)pyrazine, was synthesized by Goodwin & Lions (1959 ▸), and the crystal structures of three polymorphs have been reported; a monoclininc P21/n polymorph (VUKGAJ01; Bock et al., 1992 ▸), a tetragonal I41/a polymorph (VUKGAJ; Greaves & Stoeckli-Evans, 1992 ▸) and a second monoclinic C2/c polymorph (VUKGAJ03; Behrens & Rehder, 2009 ▸). The most recent tetrakis-substituted pyrazine ligand to be described is N,N′,N′′,N′′′-tetraethylpyrazine-2,3,5,6-tetracarboxamide (OSUTIH; Lohrman et al., 2016 ▸). In the last update of the CSD there are a total of three tetrakis-substituted thioether pyrazine compounds, viz. two polymorphs of compound 2,3,5,6-tetrakis(naphthalen-2-ylsulfanylmethyl)pyrazine (Pacifico & Stoeckli-Evans, 2004 ▸), and the ligands L1 and L2.
The role of the anion in coordination chemistry is often essential for the formation of multi-dimensional structures. The nitrate anion can be present as an isolated anion, coordinating to the metal atom or even bridging metal atoms. A search of the CSD for silver nitrate complexes yielded 2192 hits, among which it was noted that the nitrate anion can coordinate in at least 10 different manners. In the present study, three different situations are observed. In (I), the nitrate anion is present as an isolated anion. Its role here is to form C—H⋯O hydrogen bonds, resulting in the formation of a three-dimensional supramolecular structure (Fig. 5 ▸ and Table 4 ▸). In (II), the nitrate anion is essential in forming the network structure. The –Ag–L2–Ag–L2– chains, which propagate along [010], are linked by the nitrate anion in the [001] direction, so forming the metal–organic network (Fig. 6 ▸ and Table 2 ▸). Finally, there are two independent nitrate anions present in (III). They coordinate to the metal atoms in different manners, but they do not appear to be the essential elements in forming the three-dimensional framework (Fig. 7 ▸ and Table 3 ▸). Here, it is the presence of the pyridine rings, which twist about the S—Car bonds, that enables the metal atoms to cross-link, so forming the metal–organic framework.
Synthesis and crystallization
Compound (I):
A solution of L1 (50 mg, 0.16 mmol; Assoumatine & Stoeckli-Evans, 2014a ▸) in CH2Cl2 (5 ml) was introduced into a 16 mm diameter glass tube and layered with MeCN (2 ml) as a buffer zone. Then a solution of AgNO3 (27 mg, 0.16 mmol) in MeCN (5 ml) was added very gently to avoid possible mixing. The glass tube was sealed and left in the dark at room temperature for at least two weeks, whereupon yellow plate-like crystals of complex (I) were isolated at the interface between the two solutions. IR (KBr disc, cm−1): ν = 2985 w, 2912 w, 1406 bm, 1341 bs, 1141 w, 1115 w, 982 w, 828 w, 777 w, 701 vw, 478 vw.
Compound (II):
A solution of L2 (50 mg, 0.09 mmol; Assoumatine et al., 2007 ▸) in THF (5 ml) was introduced into a 16 mm diameter glass tube and layered with MeCN (2 ml) as a buffer zone. Then a solution of AgNO3 (15 mg, 0.09 mmol) in MeCN (5 ml) was added very gently to avoid possible mixing. The glass tube was sealed and left in the dark at room temperature for at least three weeks, whereupon yellow block-like crystals of complex (II) were isolated from the bottom of the tube. IR (KBr disc, cm−1): ν = 3053 vw, 2962 vw, 2927 vw, 1583 w, 1480 w, 1386 bs, 1278 vs, 1133 vw, 1023 w, 850 vw, 738 s, 690 m, 495 vw, 478 vw.
Compound (III):
A solution of L3 (50 mg, 0.09 mmol; Assoumatine & Stoeckli-Evans, 2016 ▸) in CHCl3 (5 ml) was introduced into a 16 mm diameter glass tube and layered with MeCN (2 ml) as a buffer zone. Then a solution of AgNO3 (15 mg, 0.09 mmol) in MeCN (5 ml) was added very gently to avoid possible mixing. The glass tube was sealed and left in the dark at room temperature for at least two weeks, whereupon pale-yellow needle-like crystals of complex (III) were isolated at the interface between the two solutions. IR (KBr disc, cm−1): ν = 3097 vw, 2899 vw, 1581 m, 1562 w, 1460 m, 1386 bs, 1305 bs, 1163 w, 1126 w, 1032 vw, 1004 vw, 825 vw, 759 m, 723 vw, 461vw.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 6 ▸. Complexes (I) and (II) were measured at 293 K on a four-circle diffractometer, while complex (III) was measured at 223 K on a one-circle image-plate diffractometer. In complex (I), the nitrate ion is positionally disordered and atoms O12A/O12B and O13A/O13B were refined with a fixed occupancy ratio of 0.5:0.5. No absorption correction was applied for complex (II) owing to the irregular shape of the crystal, and as there were no suitable reflections for ψ scans. For complex (III), a region of disordered electron density (25 electrons for a solvent-accessible volume of 130 Å3) was corrected for using the SQUEEZE routine in PLATON (Spek, 2015 ▸). Their formula mass and unit-cell characteristics were not taken into account for the final model. For complexes (I) and (II), only one equivalent of data were measured, hence R int = 0. In all three complexes, the H atoms were included in calculated positions and refined as riding: C—H = 0.96–0.97 Å for (I), 0.93–0.97 Å for (II) and 0.94–0.98 Å for (III), with U iso(H) = 1.5U eq(C-methyl) and 1.2U eq(C) for other H atoms.
Table 6. Experimental details.
| (I) | (II) | (III) | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | [Ag(C12H20N2S4](NO3) | [Ag2(NO3)2(C32H28N2S4)] | [Ag3(NO3)3(C28H24N6S4)] |
| M r | 490.42 | 908.56 | 1082.41 |
| Crystal system, space group | Monoclinic, P21/n | Monoclinic, P21/c | Monoclinic, C2/c |
| Temperature (K) | 293 | 293 | 223 |
| a, b, c (Å) | 10.167 (2), 13.482 (3), 13.377 (3) | 11.8437 (14), 18.5674 (14), 7.8444 (12) | 13.6319 (9), 16.2211 (10), 15.7201 (11) |
| β (°) | 100.838 (19) | 96.856 (11) | 96.607 (8) |
| V (Å3) | 1800.9 (7) | 1712.7 (4) | 3453.0 (4) |
| Z | 4 | 2 | 4 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 1.60 | 1.44 | 1.99 |
| Crystal size (mm) | 0.61 × 0.61 × 0.17 | 0.46 × 0.46 × 0.38 | 0.45 × 0.08 × 0.08 |
| Data collection | |||
| Diffractometer | Stoe AED2 4-circle | Stoe AED2 4-circle | STOE IPDS1 |
| Absorption correction | Analytical (ABST; Spek, 2009 ▸) | – | Multi-scan (MULABS; Spek, 2009 ▸) |
| T min, T max | 0.457, 0.789 | – | 0.949, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 3318, 3318, 2857 | 3178, 3178, 2606 | 13264, 3311, 1936 |
| R int | 0 | 0 | 0.072 |
| (sin θ/λ)max (Å−1) | 0.607 | 0.606 | 0.614 |
| Refinement | |||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.056, 0.161, 1.09 | 0.045, 0.100, 1.16 | 0.030, 0.052, 0.76 |
| No. of reflections | 3318 | 3178 | 3311 |
| No. of parameters | 207 | 218 | 242 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.97, −1.50 | 0.62, −0.61 | 0.43, −0.44 |
Supplementary Material
Crystal structure: contains datablock(s) I, II, III, Global. DOI: 10.1107/S2056989017002791/wm5369sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017002791/wm5369Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989017002791/wm5369IIsup3.hkl
Structure factors: contains datablock(s) III. DOI: 10.1107/S2056989017002791/wm5369IIIsup4.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
(I) catena-Poly[[silver(I)-µ-2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine] nitrate] . Crystal data
| [Ag(C12H20N2S4](NO3) | F(000) = 992 |
| Mr = 490.42 | Dx = 1.809 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.167 (2) Å | Cell parameters from 31 reflections |
| b = 13.482 (3) Å | θ = 14.1–19.7° |
| c = 13.377 (3) Å | µ = 1.60 mm−1 |
| β = 100.838 (19)° | T = 293 K |
| V = 1800.9 (7) Å3 | Plate, yellow |
| Z = 4 | 0.61 × 0.61 × 0.17 mm |
(I) catena-Poly[[silver(I)-µ-2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine] nitrate] . Data collection
| Stoe AED2 4-circle diffractometer | 2857 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.000 |
| Graphite monochromator | θmax = 25.6°, θmin = 2.2° |
| ω/2θ scans | h = −11→12 |
| Absorption correction: analytical (ABST; Spek, 2009) | k = 0→16 |
| Tmin = 0.457, Tmax = 0.789 | l = 0→16 |
| 3318 measured reflections | 2 standard reflections every 120 min |
| 3318 independent reflections | intensity decay: 5% |
(I) catena-Poly[[silver(I)-µ-2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine] nitrate] . Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.056 | H-atom parameters constrained |
| wR(F2) = 0.161 | w = 1/[σ2(Fo2) + (0.0974P)2 + 4.9525P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.09 | (Δ/σ)max < 0.001 |
| 3318 reflections | Δρmax = 1.97 e Å−3 |
| 207 parameters | Δρmin = −1.50 e Å−3 |
| 0 restraints | Extinction correction: SHELXL-2016/6 (Sheldrick 2015), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0035 (8) |
(I) catena-Poly[[silver(I)-µ-2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine] nitrate] . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
(I) catena-Poly[[silver(I)-µ-2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine] nitrate] . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Ag1 | 0.23796 (5) | 0.48987 (4) | 0.75616 (3) | 0.0401 (2) | |
| S1 | 0.43682 (14) | 0.37806 (10) | 0.73224 (10) | 0.0323 (3) | |
| S2 | 0.11067 (14) | 0.50053 (10) | 0.90707 (11) | 0.0320 (3) | |
| S3 | −0.06264 (16) | 0.24651 (10) | 0.63652 (12) | 0.0413 (4) | |
| S4 | −0.26369 (13) | 0.33208 (10) | 0.31050 (10) | 0.0330 (3) | |
| N1 | 0.4152 (4) | 0.4454 (3) | 0.9286 (3) | 0.0292 (9) | |
| N2 | 0.0779 (5) | 0.4916 (3) | 0.5959 (4) | 0.0282 (10) | |
| C1 | 0.5407 (5) | 0.4649 (4) | 0.9166 (4) | 0.0272 (11) | |
| C2 | 0.5796 (6) | 0.4261 (5) | 0.8198 (4) | 0.0360 (13) | |
| H2A | 0.645719 | 0.373887 | 0.836842 | 0.043* | |
| H2B | 0.620036 | 0.479211 | 0.787138 | 0.043* | |
| C3 | 0.4998 (7) | 0.3836 (5) | 0.6154 (5) | 0.0486 (16) | |
| H3A | 0.430481 | 0.364140 | 0.559808 | 0.073* | |
| H3B | 0.574665 | 0.339421 | 0.619518 | 0.073* | |
| H3C | 0.527752 | 0.450091 | 0.604654 | 0.073* | |
| C4 | 0.3736 (5) | 0.4814 (4) | 1.0107 (4) | 0.0268 (11) | |
| C5 | 0.2294 (5) | 0.4566 (4) | 1.0169 (4) | 0.0322 (11) | |
| H5A | 0.207925 | 0.486153 | 1.078010 | 0.039* | |
| H5B | 0.220455 | 0.385218 | 1.022356 | 0.039* | |
| C6 | 0.1099 (7) | 0.6312 (5) | 0.9332 (6) | 0.0512 (16) | |
| H6A | 0.047093 | 0.663787 | 0.880930 | 0.077* | |
| H6B | 0.197800 | 0.657895 | 0.934761 | 0.077* | |
| H6C | 0.084358 | 0.641823 | 0.997925 | 0.077* | |
| C7 | 0.0317 (5) | 0.4104 (4) | 0.5426 (4) | 0.0273 (10) | |
| C10 | −0.0478 (5) | 0.4186 (4) | 0.4465 (4) | 0.0265 (10) | |
| C11 | −0.0956 (5) | 0.3274 (4) | 0.3842 (4) | 0.0334 (12) | |
| H11A | −0.089767 | 0.271140 | 0.430044 | 0.040* | |
| H11B | −0.034496 | 0.315052 | 0.337994 | 0.040* | |
| C8 | 0.0713 (5) | 0.3120 (4) | 0.5925 (4) | 0.0313 (11) | |
| H8A | 0.143563 | 0.322787 | 0.650060 | 0.038* | |
| H8B | 0.105664 | 0.270039 | 0.544339 | 0.038* | |
| C9 | −0.0972 (9) | 0.3316 (5) | 0.7311 (6) | 0.062 (2) | |
| H9A | −0.161874 | 0.302866 | 0.766452 | 0.093* | |
| H9B | −0.132267 | 0.392150 | 0.698934 | 0.093* | |
| H9C | −0.016152 | 0.345284 | 0.778694 | 0.093* | |
| C12 | −0.3592 (7) | 0.3497 (6) | 0.4101 (6) | 0.0569 (18) | |
| H12A | −0.451820 | 0.359680 | 0.380318 | 0.085* | |
| H12B | −0.326070 | 0.406797 | 0.449937 | 0.085* | |
| H12C | −0.350633 | 0.292137 | 0.453042 | 0.085* | |
| N10 | 0.2281 (6) | 0.3702 (5) | 0.3423 (5) | 0.0544 (15) | |
| O11 | 0.2331 (7) | 0.2979 (5) | 0.3953 (5) | 0.0842 (13) | |
| O12A | 0.2834 (16) | 0.4447 (11) | 0.3565 (11) | 0.0842 (13) | 0.5 |
| O13A | 0.1631 (17) | 0.3451 (10) | 0.2526 (12) | 0.0842 (13) | 0.5 |
| O12B | 0.2588 (16) | 0.4507 (11) | 0.4035 (11) | 0.0842 (13) | 0.5 |
| O13B | 0.1847 (18) | 0.3871 (10) | 0.2537 (12) | 0.0842 (13) | 0.5 |
(I) catena-Poly[[silver(I)-µ-2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine] nitrate] . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ag1 | 0.0397 (3) | 0.0501 (3) | 0.0284 (3) | 0.00285 (18) | 0.0014 (2) | 0.00409 (17) |
| S1 | 0.0349 (7) | 0.0359 (7) | 0.0280 (7) | −0.0023 (5) | 0.0105 (5) | −0.0025 (5) |
| S2 | 0.0243 (7) | 0.0424 (8) | 0.0293 (7) | −0.0031 (5) | 0.0056 (5) | −0.0003 (5) |
| S3 | 0.0473 (9) | 0.0327 (7) | 0.0422 (8) | −0.0100 (6) | 0.0044 (7) | 0.0024 (6) |
| S4 | 0.0312 (7) | 0.0361 (7) | 0.0315 (7) | −0.0046 (5) | 0.0056 (5) | −0.0069 (5) |
| N1 | 0.022 (2) | 0.040 (2) | 0.027 (2) | −0.0010 (18) | 0.0078 (18) | −0.0002 (18) |
| N2 | 0.030 (2) | 0.029 (2) | 0.027 (2) | 0.0020 (17) | 0.0065 (19) | −0.0017 (16) |
| C1 | 0.028 (3) | 0.035 (3) | 0.020 (2) | 0.005 (2) | 0.007 (2) | 0.0033 (19) |
| C2 | 0.029 (3) | 0.054 (3) | 0.029 (3) | 0.002 (2) | 0.016 (2) | −0.006 (2) |
| C3 | 0.061 (4) | 0.056 (4) | 0.031 (3) | −0.010 (3) | 0.017 (3) | −0.011 (3) |
| C4 | 0.022 (3) | 0.037 (3) | 0.023 (2) | 0.005 (2) | 0.007 (2) | 0.004 (2) |
| C5 | 0.023 (3) | 0.041 (3) | 0.034 (3) | −0.005 (2) | 0.010 (2) | 0.005 (2) |
| C6 | 0.060 (4) | 0.038 (3) | 0.059 (4) | 0.003 (3) | 0.022 (3) | 0.004 (3) |
| C7 | 0.025 (2) | 0.028 (2) | 0.030 (3) | −0.0005 (19) | 0.008 (2) | −0.002 (2) |
| C10 | 0.024 (2) | 0.028 (2) | 0.028 (2) | 0.0012 (19) | 0.007 (2) | −0.002 (2) |
| C11 | 0.031 (3) | 0.033 (3) | 0.034 (3) | 0.000 (2) | 0.001 (2) | −0.009 (2) |
| C8 | 0.034 (3) | 0.031 (3) | 0.027 (3) | 0.001 (2) | 0.000 (2) | 0.003 (2) |
| C9 | 0.077 (5) | 0.046 (4) | 0.076 (5) | −0.009 (3) | 0.047 (4) | −0.005 (4) |
| C12 | 0.056 (4) | 0.062 (4) | 0.063 (4) | −0.006 (3) | 0.036 (4) | −0.003 (3) |
| N10 | 0.038 (3) | 0.061 (4) | 0.068 (4) | −0.006 (3) | 0.020 (3) | 0.006 (3) |
| O11 | 0.109 (4) | 0.071 (3) | 0.076 (3) | −0.016 (3) | 0.026 (3) | 0.011 (3) |
| O12A | 0.109 (4) | 0.071 (3) | 0.076 (3) | −0.016 (3) | 0.026 (3) | 0.011 (3) |
| O13A | 0.109 (4) | 0.071 (3) | 0.076 (3) | −0.016 (3) | 0.026 (3) | 0.011 (3) |
| O12B | 0.109 (4) | 0.071 (3) | 0.076 (3) | −0.016 (3) | 0.026 (3) | 0.011 (3) |
| O13B | 0.109 (4) | 0.071 (3) | 0.076 (3) | −0.016 (3) | 0.026 (3) | 0.011 (3) |
(I) catena-Poly[[silver(I)-µ-2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine] nitrate] . Geometric parameters (Å, º)
| Ag1—N1 | 2.714 (4) | C4—C5 | 1.522 (7) |
| Ag1—N2 | 2.436 (5) | C5—H5A | 0.9700 |
| Ag1—S1 | 2.5895 (15) | C5—H5B | 0.9700 |
| Ag1—S2 | 2.5987 (16) | C6—H6A | 0.9600 |
| Ag1—S4i | 2.5910 (15) | C6—H6B | 0.9600 |
| S1—C3 | 1.798 (6) | C6—H6C | 0.9600 |
| S1—C2 | 1.805 (6) | C7—C10 | 1.389 (7) |
| S2—C6 | 1.797 (7) | C7—C8 | 1.506 (7) |
| S2—C5 | 1.817 (6) | C10—C11 | 1.513 (7) |
| S3—C9 | 1.791 (7) | C11—H11A | 0.9700 |
| S3—C8 | 1.812 (6) | C11—H11B | 0.9700 |
| S4—C11 | 1.806 (5) | C8—H8A | 0.9700 |
| S4—C12 | 1.806 (7) | C8—H8B | 0.9700 |
| N1—C4 | 1.341 (7) | C9—H9A | 0.9600 |
| N1—C1 | 1.342 (7) | C9—H9B | 0.9600 |
| N2—C7 | 1.342 (7) | C9—H9C | 0.9600 |
| N2—C10i | 1.348 (6) | C12—H12A | 0.9600 |
| C1—C4ii | 1.381 (8) | C12—H12B | 0.9600 |
| C1—C2 | 1.517 (7) | C12—H12C | 0.9600 |
| C2—H2A | 0.9700 | N10—O12A | 1.148 (15) |
| C2—H2B | 0.9700 | N10—O11 | 1.202 (8) |
| C3—H3A | 0.9600 | N10—O13B | 1.205 (17) |
| C3—H3B | 0.9600 | N10—O13A | 1.301 (17) |
| C3—H3C | 0.9600 | N10—O12B | 1.360 (16) |
| N1—Ag1—N2 | 167.75 (13) | C4—C5—H5B | 109.1 |
| N1—Ag1—S1 | 64.36 (9) | S2—C5—H5B | 109.1 |
| N1—Ag1—S2 | 72.54 (9) | H5A—C5—H5B | 107.8 |
| N1—Ag1—S4i | 113.79 (9) | S2—C6—H6A | 109.5 |
| N2—Ag1—S1 | 107.74 (11) | S2—C6—H6B | 109.5 |
| N2—Ag1—S2 | 109.60 (11) | H6A—C6—H6B | 109.5 |
| N2—Ag1—S4i | 77.43 (10) | S2—C6—H6C | 109.5 |
| S1—Ag1—S2 | 129.99 (5) | H6A—C6—H6C | 109.5 |
| S1—Ag1—S4i | 111.41 (5) | H6B—C6—H6C | 109.5 |
| S4i—Ag1—S2 | 108.26 (5) | N2—C7—C10 | 120.8 (5) |
| C3—S1—C2 | 100.1 (3) | N2—C7—C8 | 116.5 (4) |
| C3—S1—Ag1 | 119.8 (2) | C10—C7—C8 | 122.8 (4) |
| C2—S1—Ag1 | 105.22 (19) | N2i—C10—C7 | 120.5 (4) |
| C6—S2—C5 | 100.9 (3) | N2i—C10—C11 | 118.3 (5) |
| C6—S2—Ag1 | 103.1 (2) | C7—C10—C11 | 121.1 (4) |
| C5—S2—Ag1 | 104.98 (18) | C10—C11—S4 | 116.4 (4) |
| C9—S3—C8 | 100.2 (3) | C10—C11—H11A | 108.2 |
| C11—S4—C12 | 100.8 (3) | S4—C11—H11A | 108.2 |
| C11—S4—Ag1i | 94.24 (19) | C10—C11—H11B | 108.2 |
| C12—S4—Ag1i | 103.6 (3) | S4—C11—H11B | 108.2 |
| C4—N1—C1 | 118.7 (5) | H11A—C11—H11B | 107.3 |
| C7—N2—C10i | 118.7 (5) | C7—C8—S3 | 114.8 (4) |
| C7—N2—Ag1 | 124.7 (3) | C7—C8—H8A | 108.6 |
| C10i—N2—Ag1 | 116.1 (3) | S3—C8—H8A | 108.6 |
| N1—C1—C4ii | 120.5 (5) | C7—C8—H8B | 108.6 |
| N1—C1—C2 | 116.1 (5) | S3—C8—H8B | 108.6 |
| C4ii—C1—C2 | 123.4 (5) | H8A—C8—H8B | 107.6 |
| C1—C2—S1 | 111.8 (4) | S3—C9—H9A | 109.5 |
| C1—C2—H2A | 109.3 | S3—C9—H9B | 109.5 |
| S1—C2—H2A | 109.3 | H9A—C9—H9B | 109.5 |
| C1—C2—H2B | 109.3 | S3—C9—H9C | 109.5 |
| S1—C2—H2B | 109.3 | H9A—C9—H9C | 109.5 |
| H2A—C2—H2B | 107.9 | H9B—C9—H9C | 109.5 |
| S1—C3—H3A | 109.5 | S4—C12—H12A | 109.5 |
| S1—C3—H3B | 109.5 | S4—C12—H12B | 109.5 |
| H3A—C3—H3B | 109.5 | H12A—C12—H12B | 109.5 |
| S1—C3—H3C | 109.5 | S4—C12—H12C | 109.5 |
| H3A—C3—H3C | 109.5 | H12A—C12—H12C | 109.5 |
| H3B—C3—H3C | 109.5 | H12B—C12—H12C | 109.5 |
| N1—C4—C1ii | 120.8 (5) | O12A—N10—O11 | 130.3 (10) |
| N1—C4—C5 | 114.9 (5) | O11—N10—O13B | 134.4 (9) |
| C1ii—C4—C5 | 124.3 (5) | O12A—N10—O13A | 121.9 (11) |
| C4—C5—S2 | 112.6 (4) | O11—N10—O13A | 106.9 (8) |
| C4—C5—H5A | 109.1 | O11—N10—O12B | 108.2 (8) |
| S2—C5—H5A | 109.1 | O13B—N10—O12B | 116.2 (10) |
| C4—N1—C1—C4ii | 1.9 (8) | C10i—N2—C7—C8 | 178.5 (5) |
| C4—N1—C1—C2 | −177.3 (5) | Ag1—N2—C7—C8 | 7.0 (6) |
| N1—C1—C2—S1 | 9.1 (6) | N2—C7—C10—N2i | 1.3 (8) |
| C4ii—C1—C2—S1 | −170.1 (4) | C8—C7—C10—N2i | −178.4 (5) |
| C3—S1—C2—C1 | 159.3 (4) | N2—C7—C10—C11 | 177.5 (5) |
| Ag1—S1—C2—C1 | 34.5 (4) | C8—C7—C10—C11 | −2.3 (8) |
| C1—N1—C4—C1ii | −1.9 (8) | N2i—C10—C11—S4 | −41.6 (6) |
| C1—N1—C4—C5 | 179.2 (5) | C7—C10—C11—S4 | 142.1 (4) |
| N1—C4—C5—S2 | −58.1 (6) | C12—S4—C11—C10 | −60.4 (5) |
| C1ii—C4—C5—S2 | 123.1 (5) | Ag1i—S4—C11—C10 | 44.3 (4) |
| C6—S2—C5—C4 | −74.8 (5) | N2—C7—C8—S3 | 107.7 (5) |
| Ag1—S2—C5—C4 | 32.1 (4) | C10—C7—C8—S3 | −72.5 (6) |
| C10i—N2—C7—C10 | −1.3 (8) | C9—S3—C8—C7 | −63.6 (5) |
| Ag1—N2—C7—C10 | −172.8 (4) |
Symmetry codes: (i) −x, −y+1, −z+1; (ii) −x+1, −y+1, −z+2.
(I) catena-Poly[[silver(I)-µ-2,3,5,6-tetrakis[(methylsulfanyl)methyl]pyrazine] nitrate] . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C9—H9C···S2 | 0.96 | 2.86 | 3.650 (8) | 141 |
| C11—H11A···S3 | 0.97 | 2.74 | 3.502 (6) | 136 |
| C11—H11B···O13A | 0.97 | 2.52 | 3.438 (17) | 157 |
| C2—H2A···O11iii | 0.97 | 2.55 | 3.460 (9) | 156 |
| C2—H2B···O12Aiv | 0.97 | 2.53 | 3.431 (15) | 154 |
| C3—H3C···O12Aiv | 0.96 | 2.37 | 3.171 (17) | 141 |
| C3—H3C···O12Biv | 0.96 | 2.57 | 3.364 (16) | 140 |
| C6—H6A···O13Ai | 0.96 | 2.52 | 3.375 (19) | 149 |
| C9—H9A···O11v | 0.96 | 2.58 | 3.503 (10) | 162 |
Symmetry codes: (i) −x, −y+1, −z+1; (iii) x+1/2, −y+1/2, z+1/2; (iv) −x+1, −y+1, −z+1; (v) x−1/2, −y+1/2, z+1/2.
(II) Poly[di-µ-nitrato-bis{µ-2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine}disilver]. Crystal data
| [Ag2(NO3)2(C32H28N2S4)] | F(000) = 908 |
| Mr = 908.56 | Dx = 1.762 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 11.8437 (14) Å | Cell parameters from 24 reflections |
| b = 18.5674 (14) Å | θ = 11.2–17.7° |
| c = 7.8444 (12) Å | µ = 1.44 mm−1 |
| β = 96.856 (11)° | T = 293 K |
| V = 1712.7 (4) Å3 | Block, pale yellow |
| Z = 2 | 0.46 × 0.46 × 0.38 mm |
(II) Poly[di-µ-nitrato-bis{µ-2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine}disilver]. Data collection
| Stoe AED2 4-circle diffractometer | Rint = 0.0 |
| Radiation source: fine-focus sealed tube | θmax = 25.5°, θmin = 2.1° |
| Graphite monochromator | h = −9→9 |
| ω/2θ scans | k = 0→22 |
| 3178 measured reflections | l = 0→14 |
| 3178 independent reflections | 2 standard reflections every 120 min |
| 2606 reflections with I > 2σ(I) | intensity decay: 2% |
(II) Poly[di-µ-nitrato-bis{µ-2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine}disilver]. Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H-atom parameters constrained |
| wR(F2) = 0.100 | w = 1/[σ2(Fo2) + (0.0308P)2 + 3.2342P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.16 | (Δ/σ)max = 0.001 |
| 3178 reflections | Δρmax = 0.62 e Å−3 |
| 218 parameters | Δρmin = −0.61 e Å−3 |
| 0 restraints | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0018 (4) |
(II) Poly[di-µ-nitrato-bis{µ-2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine}disilver]. Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
(II) Poly[di-µ-nitrato-bis{µ-2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine}disilver]. Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ag1 | 1.03726 (4) | 0.81386 (2) | 0.31389 (6) | 0.05335 (17) | |
| S1 | 0.82101 (11) | 0.85133 (7) | 0.23374 (16) | 0.0473 (3) | |
| S2 | 0.75690 (11) | 1.14870 (6) | 0.53250 (17) | 0.0458 (3) | |
| O1 | 1.0997 (4) | 0.6845 (2) | 0.2690 (5) | 0.0699 (12) | |
| O2 | 1.0189 (4) | 0.7981 (2) | −0.0056 (5) | 0.0671 (11) | |
| O3 | 1.0734 (4) | 0.5953 (2) | 0.4353 (5) | 0.0786 (13) | |
| N1 | 1.0335 (3) | 0.94469 (19) | 0.4019 (4) | 0.0368 (8) | |
| N10 | 1.0647 (4) | 0.6592 (2) | 0.4005 (5) | 0.0464 (10) | |
| C1 | 0.9323 (4) | 0.9750 (2) | 0.3633 (5) | 0.0354 (10) | |
| C2 | 0.8967 (4) | 1.0317 (2) | 0.4638 (5) | 0.0365 (10) | |
| C3 | 0.8593 (4) | 0.9458 (2) | 0.2087 (6) | 0.0435 (11) | |
| H3A | 0.8998 | 0.9505 | 0.1089 | 0.052* | |
| H3B | 0.7904 | 0.9743 | 0.1886 | 0.052* | |
| C4 | 0.7519 (4) | 0.8528 (3) | 0.4246 (6) | 0.0446 (11) | |
| C5 | 0.8133 (5) | 0.8402 (3) | 0.5828 (7) | 0.0529 (13) | |
| H5 | 0.8919 | 0.8349 | 0.5915 | 0.063* | |
| C6 | 0.7584 (6) | 0.8354 (3) | 0.7273 (7) | 0.0625 (15) | |
| H6 | 0.8002 | 0.8266 | 0.8332 | 0.075* | |
| C7 | 0.6437 (6) | 0.8435 (4) | 0.7171 (9) | 0.0738 (18) | |
| H7 | 0.6072 | 0.8402 | 0.8154 | 0.089* | |
| C8 | 0.5828 (6) | 0.8564 (5) | 0.5616 (10) | 0.091 (2) | |
| H8 | 0.5044 | 0.8621 | 0.5545 | 0.110* | |
| C9 | 0.6358 (5) | 0.8613 (4) | 0.4139 (9) | 0.0767 (19) | |
| H9 | 0.5933 | 0.8701 | 0.3084 | 0.092* | |
| C10 | 0.7834 (4) | 1.0681 (2) | 0.4170 (6) | 0.0406 (10) | |
| H10A | 0.7241 | 1.0337 | 0.4334 | 0.049* | |
| H10B | 0.7763 | 1.0797 | 0.2957 | 0.049* | |
| C11 | 0.7013 (4) | 1.1194 (3) | 0.7201 (6) | 0.0439 (11) | |
| C12 | 0.6681 (5) | 1.1731 (3) | 0.8251 (7) | 0.0588 (14) | |
| H12 | 0.6784 | 1.2211 | 0.7969 | 0.071* | |
| C13 | 0.6194 (5) | 1.1561 (4) | 0.9719 (8) | 0.0700 (17) | |
| H13 | 0.5977 | 1.1926 | 1.0422 | 0.084* | |
| C14 | 0.6031 (5) | 1.0855 (4) | 1.0141 (7) | 0.0710 (19) | |
| H14 | 0.5694 | 1.0742 | 1.1119 | 0.085* | |
| C15 | 0.6368 (5) | 1.0312 (4) | 0.9113 (7) | 0.0646 (16) | |
| H15 | 0.6262 | 0.9832 | 0.9401 | 0.078* | |
| C16 | 0.6866 (5) | 1.0481 (3) | 0.7647 (7) | 0.0539 (13) | |
| H16 | 0.7101 | 1.0115 | 0.6963 | 0.065* |
(II) Poly[di-µ-nitrato-bis{µ-2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine}disilver]. Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ag1 | 0.0616 (3) | 0.0377 (2) | 0.0624 (3) | 0.00179 (19) | 0.01433 (19) | −0.00855 (18) |
| S1 | 0.0601 (8) | 0.0426 (7) | 0.0388 (6) | −0.0055 (6) | 0.0047 (6) | −0.0088 (5) |
| S2 | 0.0516 (7) | 0.0354 (6) | 0.0513 (7) | 0.0076 (5) | 0.0109 (6) | 0.0029 (5) |
| O1 | 0.107 (3) | 0.057 (2) | 0.052 (2) | 0.015 (2) | 0.034 (2) | 0.0080 (19) |
| O2 | 0.097 (3) | 0.057 (2) | 0.052 (2) | −0.019 (2) | 0.029 (2) | −0.0007 (18) |
| O3 | 0.130 (4) | 0.040 (2) | 0.070 (3) | 0.010 (2) | 0.024 (3) | 0.0085 (19) |
| N1 | 0.047 (2) | 0.0323 (19) | 0.0303 (19) | 0.0022 (17) | 0.0034 (16) | −0.0011 (15) |
| N10 | 0.058 (3) | 0.041 (2) | 0.041 (2) | 0.003 (2) | 0.0092 (19) | 0.0019 (19) |
| C1 | 0.047 (3) | 0.028 (2) | 0.031 (2) | −0.0015 (19) | 0.0042 (19) | 0.0014 (17) |
| C2 | 0.047 (3) | 0.031 (2) | 0.032 (2) | −0.003 (2) | 0.006 (2) | 0.0035 (18) |
| C3 | 0.059 (3) | 0.037 (2) | 0.032 (2) | 0.003 (2) | −0.003 (2) | 0.0010 (19) |
| C4 | 0.050 (3) | 0.040 (3) | 0.044 (3) | −0.003 (2) | 0.005 (2) | −0.002 (2) |
| C5 | 0.046 (3) | 0.062 (3) | 0.052 (3) | 0.009 (3) | 0.009 (2) | 0.006 (3) |
| C6 | 0.078 (4) | 0.064 (4) | 0.048 (3) | 0.011 (3) | 0.015 (3) | 0.006 (3) |
| C7 | 0.079 (5) | 0.080 (4) | 0.069 (4) | −0.001 (4) | 0.035 (4) | −0.002 (3) |
| C8 | 0.049 (4) | 0.132 (7) | 0.096 (6) | −0.002 (4) | 0.022 (4) | −0.005 (5) |
| C9 | 0.054 (4) | 0.109 (6) | 0.065 (4) | −0.003 (4) | −0.005 (3) | −0.004 (4) |
| C10 | 0.048 (3) | 0.036 (2) | 0.037 (2) | 0.002 (2) | 0.004 (2) | 0.000 (2) |
| C11 | 0.040 (3) | 0.050 (3) | 0.043 (3) | −0.001 (2) | 0.006 (2) | −0.003 (2) |
| C12 | 0.065 (4) | 0.056 (3) | 0.057 (3) | 0.002 (3) | 0.013 (3) | −0.017 (3) |
| C13 | 0.066 (4) | 0.090 (5) | 0.055 (4) | 0.002 (4) | 0.011 (3) | −0.019 (3) |
| C14 | 0.052 (3) | 0.122 (6) | 0.041 (3) | −0.012 (4) | 0.010 (3) | 0.001 (3) |
| C15 | 0.060 (4) | 0.079 (4) | 0.055 (3) | −0.016 (3) | 0.009 (3) | 0.012 (3) |
| C16 | 0.060 (3) | 0.052 (3) | 0.051 (3) | −0.002 (3) | 0.013 (3) | −0.001 (2) |
(II) Poly[di-µ-nitrato-bis{µ-2,3,5,6-tetrakis[(phenylsulfanyl)methyl]pyrazine}disilver]. Geometric parameters (Å, º)
| Ag1—N1 | 2.527 (4) | C4—C9 | 1.376 (8) |
| Ag1—S1 | 2.6560 (15) | C4—C5 | 1.382 (7) |
| Ag1—S2i | 2.6790 (14) | C5—C6 | 1.376 (7) |
| Ag1—O1 | 2.551 (4) | C5—H5 | 0.9300 |
| Ag1—O2 | 2.507 (4) | C6—C7 | 1.359 (9) |
| Ag1—O2ii | 2.539 (4) | C6—H6 | 0.9300 |
| S1—C4 | 1.790 (5) | C7—C8 | 1.363 (10) |
| S1—C3 | 1.828 (5) | C7—H7 | 0.9300 |
| S2—C11 | 1.768 (5) | C8—C9 | 1.385 (9) |
| S2—C10 | 1.796 (4) | C8—H8 | 0.9300 |
| S2—Ag1i | 2.6790 (14) | C9—H9 | 0.9300 |
| O1—N10 | 1.248 (5) | C10—H10A | 0.9700 |
| O2—N10iii | 1.250 (5) | C10—H10B | 0.9700 |
| O2—Ag1iii | 2.539 (4) | C11—C12 | 1.381 (7) |
| O3—N10 | 1.219 (5) | C11—C16 | 1.385 (7) |
| N1—C1 | 1.326 (6) | C12—C13 | 1.385 (8) |
| N1—C2i | 1.334 (5) | C12—H12 | 0.9300 |
| N10—O2ii | 1.250 (5) | C13—C14 | 1.372 (9) |
| C1—C2 | 1.409 (6) | C13—H13 | 0.9300 |
| C1—C3 | 1.504 (6) | C14—C15 | 1.380 (9) |
| C2—N1i | 1.334 (5) | C14—H14 | 0.9300 |
| C2—C10 | 1.508 (6) | C15—C16 | 1.390 (7) |
| C3—H3A | 0.9700 | C15—H15 | 0.9300 |
| C3—H3B | 0.9700 | C16—H16 | 0.9300 |
| N1—Ag1—S1 | 76.40 (9) | C9—C4—C5 | 119.3 (5) |
| N1—Ag1—S2i | 70.89 (9) | C9—C4—S1 | 120.3 (4) |
| S1—Ag1—S2i | 146.98 (4) | C5—C4—S1 | 120.2 (4) |
| O2—Ag1—N1 | 112.54 (12) | C6—C5—C4 | 120.1 (5) |
| O2—Ag1—O2ii | 117.32 (8) | C6—C5—H5 | 120.0 |
| N1—Ag1—O2ii | 128.98 (12) | C4—C5—H5 | 120.0 |
| O2—Ag1—O1 | 75.15 (13) | C7—C6—C5 | 120.8 (6) |
| N1—Ag1—O1 | 163.34 (14) | C7—C6—H6 | 119.6 |
| O2ii—Ag1—O1 | 49.56 (12) | C5—C6—H6 | 119.6 |
| O2—Ag1—S1 | 80.10 (11) | C6—C7—C8 | 119.4 (6) |
| O2ii—Ag1—S1 | 101.67 (11) | C6—C7—H7 | 120.3 |
| O1—Ag1—S1 | 120.09 (11) | C8—C7—H7 | 120.3 |
| O2—Ag1—S2i | 116.46 (10) | C7—C8—C9 | 121.1 (6) |
| O2ii—Ag1—S2i | 95.47 (11) | C7—C8—H8 | 119.5 |
| O1—Ag1—S2i | 92.47 (11) | C9—C8—H8 | 119.5 |
| C4—S1—C3 | 102.6 (2) | C4—C9—C8 | 119.4 (6) |
| C4—S1—Ag1 | 109.31 (17) | C4—C9—H9 | 120.3 |
| C3—S1—Ag1 | 91.85 (17) | C8—C9—H9 | 120.3 |
| C11—S2—C10 | 105.6 (2) | C2—C10—S2 | 117.1 (3) |
| C11—S2—Ag1i | 96.58 (16) | C2—C10—H10A | 108.0 |
| C10—S2—Ag1i | 103.79 (16) | S2—C10—H10A | 108.0 |
| N10—O1—Ag1 | 96.3 (3) | C2—C10—H10B | 108.0 |
| N10iii—O2—Ag1 | 121.5 (3) | S2—C10—H10B | 108.0 |
| N10iii—O2—Ag1iii | 96.8 (3) | H10A—C10—H10B | 107.3 |
| Ag1—O2—Ag1iii | 130.58 (17) | C12—C11—C16 | 119.1 (5) |
| C1—N1—C2i | 119.9 (4) | C12—C11—S2 | 115.8 (4) |
| C1—N1—Ag1 | 113.0 (3) | C16—C11—S2 | 125.1 (4) |
| C2i—N1—Ag1 | 120.2 (3) | C11—C12—C13 | 120.5 (6) |
| O3—N10—O1 | 121.6 (4) | C11—C12—H12 | 119.8 |
| O3—N10—O2ii | 121.1 (4) | C13—C12—H12 | 119.8 |
| O1—N10—O2ii | 117.3 (4) | C14—C13—C12 | 120.3 (6) |
| N1—C1—C2 | 120.8 (4) | C14—C13—H13 | 119.9 |
| N1—C1—C3 | 116.6 (4) | C12—C13—H13 | 119.9 |
| C2—C1—C3 | 122.6 (4) | C13—C14—C15 | 119.9 (5) |
| N1i—C2—C1 | 119.2 (4) | C13—C14—H14 | 120.0 |
| N1i—C2—C10 | 119.6 (4) | C15—C14—H14 | 120.0 |
| C1—C2—C10 | 121.1 (4) | C14—C15—C16 | 119.9 (6) |
| C1—C3—S1 | 112.7 (3) | C14—C15—H15 | 120.0 |
| C1—C3—H3A | 109.1 | C16—C15—H15 | 120.0 |
| S1—C3—H3A | 109.1 | C11—C16—C15 | 120.3 (5) |
| C1—C3—H3B | 109.1 | C11—C16—H16 | 119.8 |
| S1—C3—H3B | 109.1 | C15—C16—H16 | 119.8 |
| H3A—C3—H3B | 107.8 | ||
| Ag1—O1—N10—O3 | 178.4 (5) | C5—C6—C7—C8 | 0.0 (10) |
| Ag1—O1—N10—O2ii | −0.7 (5) | C6—C7—C8—C9 | 0.2 (12) |
| C2i—N1—C1—C2 | −0.7 (7) | C5—C4—C9—C8 | −0.4 (10) |
| Ag1—N1—C1—C2 | 150.3 (3) | S1—C4—C9—C8 | 174.8 (6) |
| C2i—N1—C1—C3 | 179.3 (4) | C7—C8—C9—C4 | 0.0 (12) |
| Ag1—N1—C1—C3 | −29.7 (5) | N1i—C2—C10—S2 | 7.1 (6) |
| N1—C1—C2—N1i | 0.7 (7) | C1—C2—C10—S2 | −170.1 (3) |
| C3—C1—C2—N1i | −179.3 (4) | C11—S2—C10—C2 | −86.4 (4) |
| N1—C1—C2—C10 | 177.9 (4) | Ag1i—S2—C10—C2 | 14.6 (4) |
| C3—C1—C2—C10 | −2.2 (6) | C10—S2—C11—C12 | −176.3 (4) |
| N1—C1—C3—S1 | 62.9 (5) | Ag1i—S2—C11—C12 | 77.3 (4) |
| C2—C1—C3—S1 | −117.1 (4) | C10—S2—C11—C16 | 1.8 (5) |
| C4—S1—C3—C1 | 57.2 (4) | Ag1i—S2—C11—C16 | −104.5 (5) |
| Ag1—S1—C3—C1 | −53.1 (3) | C16—C11—C12—C13 | −0.7 (8) |
| C3—S1—C4—C9 | 92.2 (5) | S2—C11—C12—C13 | 177.6 (5) |
| Ag1—S1—C4—C9 | −171.3 (5) | C11—C12—C13—C14 | −0.4 (9) |
| C3—S1—C4—C5 | −92.6 (5) | C12—C13—C14—C15 | 1.0 (10) |
| Ag1—S1—C4—C5 | 3.9 (5) | C13—C14—C15—C16 | −0.4 (9) |
| C9—C4—C5—C6 | 0.6 (9) | C12—C11—C16—C15 | 1.3 (8) |
| S1—C4—C5—C6 | −174.6 (4) | S2—C11—C16—C15 | −176.8 (4) |
| C4—C5—C6—C7 | −0.4 (9) | C14—C15—C16—C11 | −0.8 (9) |
Symmetry codes: (i) −x+2, −y+2, −z+1; (ii) x, −y+3/2, z+1/2; (iii) x, −y+3/2, z−1/2.
(III) Poly[[trinitrato{µ6-2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine}trisilver(I)] . Crystal data
| [Ag3(NO3)3(C28H24N6S4)] | F(000) = 2128 |
| Mr = 1082.41 | Dx = 2.082 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 13.6319 (9) Å | Cell parameters from 5000 reflections |
| b = 16.2211 (10) Å | θ = 2.0–25.9° |
| c = 15.7201 (11) Å | µ = 1.99 mm−1 |
| β = 96.607 (8)° | T = 223 K |
| V = 3453.0 (4) Å3 | Needle, pale yellow |
| Z = 4 | 0.45 × 0.08 × 0.08 mm |
(III) Poly[[trinitrato{µ6-2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine}trisilver(I)] . Data collection
| STOE IPDS 1 diffractometer | 3311 independent reflections |
| Radiation source: fine-focus sealed tube | 1936 reflections with I > 2σ(I) |
| Plane graphite monochromator | Rint = 0.072 |
| φ rotation scans | θmax = 25.9°, θmin = 2.0° |
| Absorption correction: multi-scan (MULABS; Spek, 2009) | h = −16→16 |
| Tmin = 0.949, Tmax = 1.000 | k = −19→19 |
| 13264 measured reflections | l = −19→18 |
(III) Poly[[trinitrato{µ6-2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine}trisilver(I)] . Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.030 | H-atom parameters constrained |
| wR(F2) = 0.052 | w = 1/[σ2(Fo2) + (0.0179P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.76 | (Δ/σ)max < 0.001 |
| 3311 reflections | Δρmax = 0.43 e Å−3 |
| 242 parameters | Δρmin = −0.44 e Å−3 |
| 0 restraints | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.00014 (3) |
(III) Poly[[trinitrato{µ6-2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine}trisilver(I)] . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
(III) Poly[[trinitrato{µ6-2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine}trisilver(I)] . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ag1 | 0.01942 (2) | 0.37808 (2) | −0.08024 (3) | 0.04096 (12) | |
| Ag2 | 0.5000 | −0.03325 (3) | 0.2500 | 0.05015 (18) | |
| S1 | 0.09150 (7) | 0.43818 (6) | 0.08153 (8) | 0.0309 (3) | |
| S2 | 0.37324 (7) | 0.12904 (6) | 0.20654 (7) | 0.0293 (3) | |
| O11 | 0.0033 (3) | 0.2151 (3) | −0.1140 (3) | 0.0993 (17) | |
| O12 | 0.0505 (3) | 0.1241 (2) | −0.0186 (3) | 0.0881 (13) | |
| O13 | −0.0038 (3) | 0.2406 (3) | 0.0181 (3) | 0.0871 (14) | |
| O21 | 0.5000 | −0.1915 (3) | 0.2500 | 0.082 (2) | |
| O22 | 0.5755 (3) | −0.3025 (3) | 0.2419 (3) | 0.0890 (15) | |
| N1 | 0.1890 (2) | 0.31443 (19) | −0.0280 (2) | 0.0228 (8) | |
| N2 | 0.1418 (2) | 0.59202 (19) | 0.0729 (2) | 0.0278 (8) | |
| N3 | 0.3466 (2) | −0.0278 (2) | 0.1875 (2) | 0.0277 (8) | |
| N11 | 0.0173 (3) | 0.1915 (3) | −0.0375 (4) | 0.0643 (14) | |
| N21 | 0.5000 | −0.2669 (3) | 0.2500 | 0.0365 (13) | |
| C1 | 0.2093 (3) | 0.2984 (2) | 0.0553 (3) | 0.0232 (9) | |
| C2 | 0.2715 (3) | 0.2341 (2) | 0.0833 (3) | 0.0217 (9) | |
| C3 | 0.1631 (3) | 0.3497 (2) | 0.1204 (3) | 0.0306 (11) | |
| H3A | 0.2161 | 0.3689 | 0.1632 | 0.037* | |
| H3B | 0.1204 | 0.3136 | 0.1499 | 0.037* | |
| C4 | 0.1816 (3) | 0.5165 (2) | 0.0793 (3) | 0.0295 (10) | |
| C5 | 0.2821 (3) | 0.5034 (3) | 0.0844 (3) | 0.0517 (14) | |
| H5 | 0.3082 | 0.4497 | 0.0867 | 0.062* | |
| C6 | 0.3433 (3) | 0.5714 (3) | 0.0861 (4) | 0.0617 (16) | |
| H6 | 0.4122 | 0.5645 | 0.0912 | 0.074* | |
| C7 | 0.3033 (3) | 0.6488 (3) | 0.0803 (3) | 0.0518 (14) | |
| H7 | 0.3442 | 0.6956 | 0.0814 | 0.062* | |
| C8 | 0.2039 (3) | 0.6569 (3) | 0.0731 (3) | 0.0407 (12) | |
| H8 | 0.1768 | 0.7102 | 0.0679 | 0.049* | |
| C9 | 0.2962 (3) | 0.2171 (2) | 0.1786 (3) | 0.0279 (10) | |
| H9A | 0.2341 | 0.2099 | 0.2036 | 0.034* | |
| H9B | 0.3290 | 0.2660 | 0.2053 | 0.034* | |
| C11 | 0.2968 (3) | −0.0982 (3) | 0.1679 (3) | 0.0406 (12) | |
| H11 | 0.3317 | −0.1483 | 0.1722 | 0.049* | |
| C12 | 0.1982 (4) | −0.0998 (3) | 0.1421 (3) | 0.0568 (15) | |
| H12 | 0.1653 | −0.1501 | 0.1296 | 0.068* | |
| C13 | 0.1476 (4) | −0.0263 (3) | 0.1347 (4) | 0.0654 (17) | |
| H13 | 0.0792 | −0.0260 | 0.1174 | 0.079* | |
| C14 | 0.1966 (3) | 0.0468 (3) | 0.1524 (3) | 0.0492 (13) | |
| H14 | 0.1631 | 0.0976 | 0.1466 | 0.059* | |
| C15 | 0.2959 (3) | 0.0433 (2) | 0.1789 (3) | 0.0276 (10) |
(III) Poly[[trinitrato{µ6-2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine}trisilver(I)] . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ag1 | 0.02369 (17) | 0.0330 (2) | 0.0673 (3) | 0.00667 (16) | 0.01013 (16) | 0.0075 (2) |
| Ag2 | 0.0223 (3) | 0.0276 (3) | 0.0975 (5) | 0.000 | −0.0062 (3) | 0.000 |
| S1 | 0.0246 (5) | 0.0246 (6) | 0.0447 (7) | 0.0066 (5) | 0.0092 (5) | 0.0023 (5) |
| S2 | 0.0227 (5) | 0.0279 (6) | 0.0354 (6) | 0.0041 (5) | −0.0043 (5) | 0.0031 (6) |
| O11 | 0.080 (3) | 0.112 (4) | 0.097 (4) | −0.053 (3) | −0.026 (3) | 0.032 (3) |
| O12 | 0.058 (2) | 0.044 (2) | 0.160 (4) | 0.010 (2) | 0.000 (2) | 0.016 (3) |
| O13 | 0.053 (2) | 0.061 (3) | 0.154 (4) | −0.013 (2) | 0.037 (3) | −0.001 (3) |
| O21 | 0.106 (4) | 0.025 (3) | 0.129 (5) | 0.000 | 0.077 (4) | 0.000 |
| O22 | 0.070 (3) | 0.100 (3) | 0.105 (3) | 0.054 (3) | 0.041 (3) | 0.036 (3) |
| N1 | 0.0195 (17) | 0.0221 (18) | 0.027 (2) | 0.0040 (14) | 0.0037 (16) | 0.0043 (16) |
| N2 | 0.0240 (18) | 0.0266 (19) | 0.033 (2) | 0.0020 (15) | 0.0058 (16) | −0.0011 (16) |
| N3 | 0.0243 (18) | 0.0279 (19) | 0.030 (2) | −0.0017 (16) | 0.0012 (16) | 0.0009 (18) |
| N11 | 0.030 (2) | 0.048 (3) | 0.114 (5) | −0.015 (2) | 0.004 (3) | −0.004 (4) |
| N21 | 0.032 (3) | 0.037 (3) | 0.041 (4) | 0.000 | 0.005 (3) | 0.000 |
| C1 | 0.017 (2) | 0.018 (2) | 0.034 (3) | 0.0004 (16) | 0.0019 (19) | 0.000 (2) |
| C2 | 0.017 (2) | 0.018 (2) | 0.030 (3) | 0.0008 (16) | 0.0046 (19) | 0.005 (2) |
| C3 | 0.034 (2) | 0.029 (2) | 0.031 (3) | 0.0115 (19) | 0.016 (2) | 0.006 (2) |
| C4 | 0.022 (2) | 0.035 (3) | 0.031 (3) | 0.0032 (19) | 0.0019 (19) | 0.000 (2) |
| C5 | 0.033 (3) | 0.043 (3) | 0.082 (4) | 0.010 (2) | 0.014 (3) | −0.001 (3) |
| C6 | 0.026 (3) | 0.062 (4) | 0.098 (5) | −0.002 (3) | 0.012 (3) | −0.002 (3) |
| C7 | 0.036 (3) | 0.050 (3) | 0.070 (4) | −0.010 (2) | 0.008 (3) | −0.009 (3) |
| C8 | 0.037 (3) | 0.034 (3) | 0.053 (3) | 0.001 (2) | 0.010 (2) | 0.000 (2) |
| C9 | 0.026 (2) | 0.023 (2) | 0.034 (3) | 0.0067 (18) | 0.000 (2) | 0.000 (2) |
| C11 | 0.041 (3) | 0.032 (3) | 0.048 (3) | −0.005 (2) | −0.003 (2) | 0.003 (2) |
| C12 | 0.048 (3) | 0.044 (3) | 0.072 (4) | −0.015 (3) | −0.020 (3) | 0.006 (3) |
| C13 | 0.034 (3) | 0.054 (4) | 0.100 (5) | −0.007 (3) | −0.026 (3) | 0.018 (3) |
| C14 | 0.032 (3) | 0.038 (3) | 0.074 (4) | 0.006 (2) | −0.010 (2) | 0.014 (3) |
| C15 | 0.025 (2) | 0.030 (2) | 0.028 (3) | 0.0002 (19) | 0.0030 (19) | 0.006 (2) |
(III) Poly[[trinitrato{µ6-2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine}trisilver(I)] . Geometric parameters (Å, º)
| Ag1—N1 | 2.578 (3) | N21—O22iii | 1.200 (4) |
| Ag1—N2i | 2.267 (3) | C1—C2 | 1.384 (5) |
| Ag1—S1 | 2.7943 (13) | C1—C3 | 1.512 (5) |
| Ag1—S2ii | 2.6010 (11) | C2—N1ii | 1.331 (5) |
| Ag1—O11 | 2.700 (5) | C2—C9 | 1.523 (5) |
| Ag1—O13 | 2.752 (5) | C3—H3A | 0.9800 |
| Ag2—N3 | 2.208 (3) | C3—H3B | 0.9800 |
| Ag2—N3iii | 2.208 (3) | C4—C5 | 1.379 (6) |
| Ag2—O21 | 2.567 (5) | C5—C6 | 1.382 (6) |
| S1—C4 | 1.770 (4) | C5—H5 | 0.9400 |
| S1—C3 | 1.802 (4) | C6—C7 | 1.368 (6) |
| S2—C15 | 1.769 (4) | C6—H6 | 0.9400 |
| S2—C9 | 1.798 (4) | C7—C8 | 1.353 (6) |
| S2—Ag1ii | 2.6011 (11) | C7—H7 | 0.9400 |
| O11—N11 | 1.255 (6) | C8—H8 | 0.9400 |
| O12—N11 | 1.208 (5) | C9—H9A | 0.9800 |
| O13—N11 | 1.239 (6) | C9—H9B | 0.9800 |
| O21—N21 | 1.223 (6) | C11—C12 | 1.360 (6) |
| O22—N21 | 1.200 (4) | C11—H11 | 0.9400 |
| N1—C2ii | 1.331 (5) | C12—C13 | 1.377 (6) |
| N1—C1 | 1.332 (5) | C12—H12 | 0.9400 |
| N2—C4 | 1.339 (5) | C13—C14 | 1.374 (6) |
| N2—C8 | 1.351 (5) | C13—H13 | 0.9400 |
| N2—Ag1i | 2.266 (3) | C14—C15 | 1.372 (6) |
| N3—C15 | 1.343 (5) | C14—H14 | 0.9400 |
| N3—C11 | 1.345 (5) | ||
| N1—Ag1—N2i | 155.31 (11) | N1ii—C2—C9 | 118.4 (3) |
| S1—Ag1—S2ii | 122.71 (3) | C1—C2—C9 | 120.4 (4) |
| S1—Ag1—N1 | 68.98 (7) | C1—C3—S1 | 117.4 (3) |
| S1—Ag1—N2i | 96.92 (8) | C1—C3—H3A | 107.9 |
| S2ii—Ag1—N1 | 70.29 (7) | S1—C3—H3A | 107.9 |
| S2ii—Ag1—N2i | 133.03 (8) | C1—C3—H3B | 107.9 |
| S1—Ag1—O11 | 122.18 (10) | S1—C3—H3B | 107.9 |
| S1—Ag1—O13 | 79.78 (10) | H3A—C3—H3B | 107.2 |
| S2ii—Ag1—O11 | 81.18 (10) | N2—C4—C5 | 122.4 (4) |
| S2ii—Ag1—O13 | 120.26 (10) | N2—C4—S1 | 112.5 (3) |
| O11—Ag1—N1 | 73.76 (11) | C5—C4—S1 | 125.1 (3) |
| O11—Ag1—N2i | 99.33 (12) | C4—C5—C6 | 118.2 (4) |
| O13—Ag1—N1 | 69.73 (11) | C4—C5—H5 | 120.9 |
| O13—Ag1—N2i | 88.28 (12) | C6—C5—H5 | 120.9 |
| O11—Ag1—O13 | 45.99 (14) | C7—C6—C5 | 119.7 (4) |
| N3—Ag2—N3iii | 175.41 (12) | C7—C6—H6 | 120.1 |
| O21—Ag2—N3 | 92.30 (9) | C5—C6—H6 | 120.1 |
| O21—Ag2—N3iii | 92.30 (9) | C8—C7—C6 | 118.8 (4) |
| C4—S1—C3 | 103.19 (19) | C8—C7—H7 | 120.6 |
| C4—S1—Ag1 | 113.84 (14) | C6—C7—H7 | 120.6 |
| C3—S1—Ag1 | 98.68 (14) | N2—C8—C7 | 123.1 (4) |
| C15—S2—C9 | 104.50 (18) | N2—C8—H8 | 118.4 |
| C15—S2—Ag1ii | 98.56 (13) | C7—C8—H8 | 118.4 |
| C9—S2—Ag1ii | 102.31 (13) | C2—C9—S2 | 116.1 (3) |
| N21—O21—Ag2 | 180.0 | C2—C9—H9A | 108.3 |
| C2ii—N1—C1 | 118.2 (3) | S2—C9—H9A | 108.3 |
| C2ii—N1—Ag1 | 116.4 (3) | C2—C9—H9B | 108.3 |
| C1—N1—Ag1 | 118.0 (2) | S2—C9—H9B | 108.3 |
| C4—N2—C8 | 117.6 (3) | H9A—C9—H9B | 107.4 |
| C4—N2—Ag1i | 125.4 (2) | N3—C11—C12 | 122.7 (4) |
| C8—N2—Ag1i | 116.4 (3) | N3—C11—H11 | 118.6 |
| C15—N3—C11 | 117.8 (3) | C12—C11—H11 | 118.6 |
| C15—N3—Ag2 | 121.9 (3) | C11—C12—C13 | 118.5 (4) |
| C11—N3—Ag2 | 119.7 (3) | C11—C12—H12 | 120.8 |
| O12—N11—O13 | 121.3 (6) | C13—C12—H12 | 120.8 |
| O12—N11—O11 | 121.5 (6) | C14—C13—C12 | 120.3 (4) |
| O13—N11—O11 | 117.2 (6) | C14—C13—H13 | 119.9 |
| O22—N21—O22iii | 122.4 (6) | C12—C13—H13 | 119.9 |
| O22—N21—O21 | 118.8 (3) | C15—C14—C13 | 117.7 (4) |
| O22iii—N21—O21 | 118.8 (3) | C15—C14—H14 | 121.1 |
| N1—C1—C2 | 120.7 (3) | C13—C14—H14 | 121.1 |
| N1—C1—C3 | 120.1 (3) | N3—C15—C14 | 123.0 (4) |
| C2—C1—C3 | 119.2 (4) | N3—C15—S2 | 111.5 (3) |
| N1ii—C2—C1 | 121.1 (4) | C14—C15—S2 | 125.5 (3) |
| C2ii—N1—C1—C2 | 1.2 (6) | C5—C6—C7—C8 | 0.0 (8) |
| Ag1—N1—C1—C2 | 150.0 (3) | C4—N2—C8—C7 | −0.8 (6) |
| C2ii—N1—C1—C3 | −177.7 (3) | Ag1i—N2—C8—C7 | 171.1 (4) |
| Ag1—N1—C1—C3 | −28.9 (4) | C6—C7—C8—N2 | 1.4 (8) |
| N1—C1—C2—N1ii | −1.3 (6) | N1ii—C2—C9—S2 | −2.7 (5) |
| C3—C1—C2—N1ii | 177.7 (3) | C1—C2—C9—S2 | 177.3 (3) |
| N1—C1—C2—C9 | 178.7 (3) | C15—S2—C9—C2 | −72.0 (3) |
| C3—C1—C2—C9 | −2.3 (5) | Ag1ii—S2—C9—C2 | 30.3 (3) |
| N1—C1—C3—S1 | −6.3 (5) | C15—N3—C11—C12 | −1.6 (6) |
| C2—C1—C3—S1 | 174.7 (3) | Ag2—N3—C11—C12 | 169.3 (4) |
| C4—S1—C3—C1 | −85.5 (3) | N3—C11—C12—C13 | 0.9 (7) |
| Ag1—S1—C3—C1 | 31.6 (3) | C11—C12—C13—C14 | 0.6 (8) |
| C8—N2—C4—C5 | −1.0 (6) | C12—C13—C14—C15 | −1.2 (8) |
| Ag1i—N2—C4—C5 | −172.2 (3) | C11—N3—C15—C14 | 0.9 (6) |
| C8—N2—C4—S1 | 178.2 (3) | Ag2—N3—C15—C14 | −169.7 (3) |
| Ag1i—N2—C4—S1 | 7.1 (4) | C11—N3—C15—S2 | −179.5 (3) |
| C3—S1—C4—N2 | −164.1 (3) | Ag2—N3—C15—S2 | 9.8 (4) |
| Ag1—S1—C4—N2 | 90.0 (3) | C13—C14—C15—N3 | 0.4 (7) |
| C3—S1—C4—C5 | 15.1 (5) | C13—C14—C15—S2 | −179.1 (4) |
| Ag1—S1—C4—C5 | −90.8 (4) | C9—S2—C15—N3 | 173.2 (3) |
| N2—C4—C5—C6 | 2.3 (7) | Ag1ii—S2—C15—N3 | 68.1 (3) |
| S1—C4—C5—C6 | −176.8 (4) | C9—S2—C15—C14 | −7.2 (4) |
| C4—C5—C6—C7 | −1.7 (8) | Ag1ii—S2—C15—C14 | −112.4 (4) |
Symmetry codes: (i) −x, −y+1, −z; (ii) −x+1/2, −y+1/2, −z; (iii) −x+1, y, −z+1/2.
(III) Poly[[trinitrato{µ6-2,3,5,6-tetrakis[(pyridin-2-ylsulfanyl)methyl]pyrazine}trisilver(I)] . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C11—H11···O21 | 0.94 | 2.57 | 3.287 (5) | 133 |
| C3—H3B···O21iv | 0.98 | 2.40 | 3.253 (4) | 145 |
| C3—H3B···O22iv | 0.98 | 2.49 | 3.420 (6) | 158 |
| C7—H7···O13v | 0.94 | 2.51 | 3.268 (6) | 138 |
| C9—H9A···O22iv | 0.98 | 2.32 | 3.291 (6) | 171 |
| C12—H12···O11vi | 0.94 | 2.51 | 3.310 (7) | 142 |
| C14—H14···O22iv | 0.94 | 2.59 | 3.349 (7) | 138 |
Symmetry codes: (iv) x−1/2, y+1/2, z; (v) x+1/2, y+1/2, z; (vi) −x, −y, −z.
Funding Statement
This work was funded by Swiss National Science Foundation grant . University of Neuchâtel grant .
References
- Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J. & Verschoor, G. C. (1984). J. Chem. Soc. Dalton Trans. pp. 1349–1356.
- Assoumatine, T. (1999). PhD thesis, University of Neuchâtel, Switzerland.
- Assoumatine, T., Gasser, G. & Stoeckli-Evans, H. (2007). Acta Cryst. C63, o219–o222. [DOI] [PubMed]
- Assoumatine, T. & Stoeckli-Evans, H. (2014a). Acta Cryst. E70, 51–53. [DOI] [PMC free article] [PubMed]
- Assoumatine, T. & Stoeckli-Evans, H. (2014b). Acta Cryst. E70, o887–o888. [DOI] [PMC free article] [PubMed]
- Assoumatine, T. & Stoeckli-Evans, H. (2016). IUCrData, 1, x161977.
- Behrens, A. & Rehder, D. (2009). Private Communication (CCDC No. 261615, refcode VUKGAJ03). CCDC, Cambridge, England.
- Black, C. A. & Hanton, L. R. (2007). Cryst. Growth Des. 7, 1868–1871.
- Bock, H., Vaupel, T., Näther, C., Ruppert, K. & Havlas, Z. (1992). Angew. Chem. Int. Ed. Engl. 31, 299–301.
- Caradoc-Davies, P. L. & Hanton, L. R. (2001). Chem. Commun. pp. 1098–1099.
- Caradoc-Davies, P. L., Hanton, L. R. & Henderson, W. (2001). J. Chem. Soc. Dalton Trans. pp. 2749–2755.
- Goodwin, H. A. & Lions, F. (1959). J. Am. Chem. Soc. 81, 6415–6422.
- Greaves, B. & Stoeckli-Evans, H. (1992). Acta Cryst. C48, 2269–2271.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Lohrman, J., Telikepalli, H., Johnson, T. S., Jackson, T. A., Day, V. W. & Bowman-James, K. (2016). Inorg. Chem. 55, 5098–5100. [DOI] [PubMed]
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Pacifico, J. & Stoeckli-Evans, H. (2004). Acta Cryst. C60, o152–o155. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Spek, A. L. (2015). Acta Cryst. C71, 9–18. [DOI] [PubMed]
- Stoe & Cie (1997). STADI4 and X-RED. Stoe & Cie GmbH, Darmstadt, Germany.
- Stoe & Cie (1998). IPDS-I Bedienungshandbuch. Stoe & Cie GmbH, Darmstadt, Germany.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, II, III, Global. DOI: 10.1107/S2056989017002791/wm5369sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017002791/wm5369Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989017002791/wm5369IIsup3.hkl
Structure factors: contains datablock(s) III. DOI: 10.1107/S2056989017002791/wm5369IIIsup4.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report









