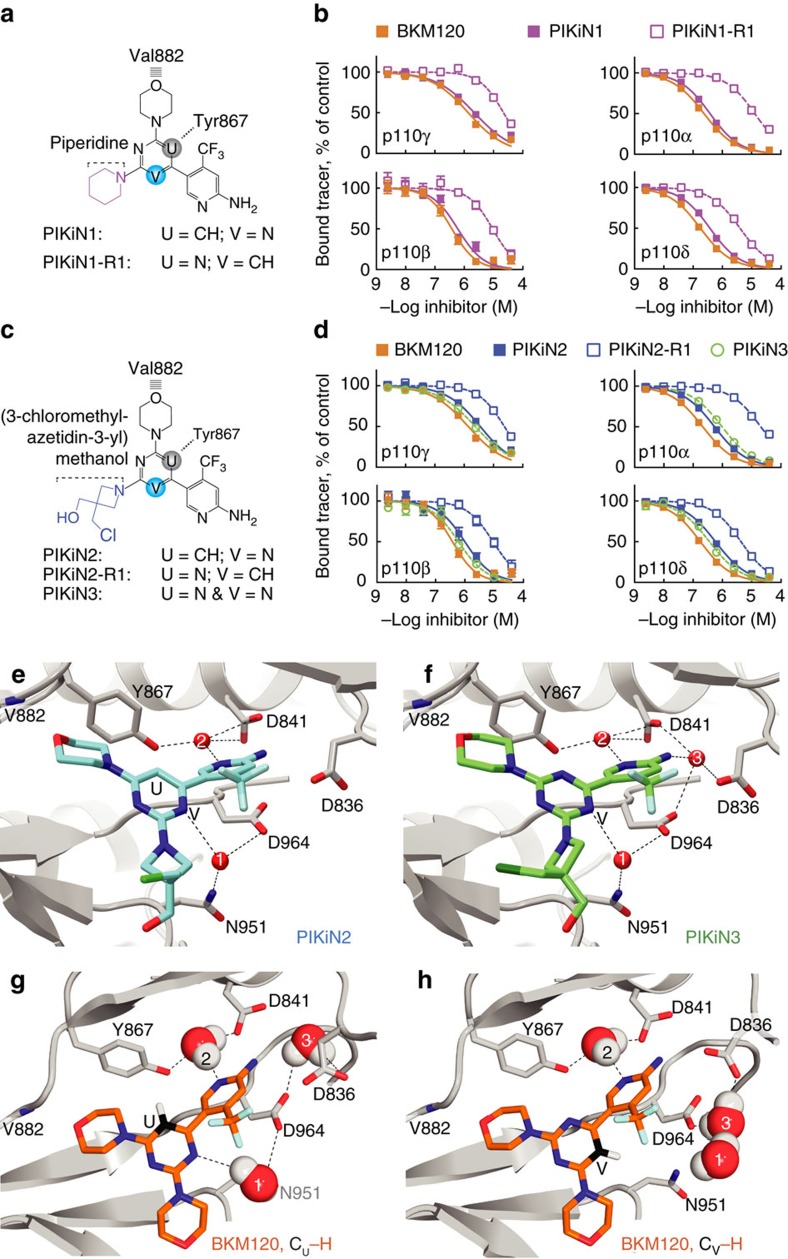
Figure 5. Binding of asymmetric BKM120 derivatives to PI3Ks.
(a,c) Chemical formulas of regioisomeric pairs. Val882 and Tyr867 of p110γ indicate schematically the drugs orientations. (b,d) PI3K isoform-specific competitive binding assays used for Kd calculations in Supplementary Table 4 (n≥2 × 2 (details in Methods); error bars omitted when smaller than symbols). (e,f) Co-crystal structure of p110γ soaked with PIKiN2 (e, (PDB ID 5JHA)) and PIKiN3 (f, (PDB ID 5JHB)), depicting structured water molecules (red numbered spheres), and water coordinating amino acids with hydrogen bonds as dashed lines. (g,h) Two opposing orientations of BKM120 in p110γ were set as starting points for modelling of water movements. Positions of water dipoles after molecular dynamics calculations and energy minimization are shown. The BKM120 structure (from PDB ID 3SD5) was fitted into the protein/water scaffold of the PIKiN3–p110γ complex before molecular dynamics calculations. Water molecules, but not BKM120 and protein, were allowed to move during calculations.