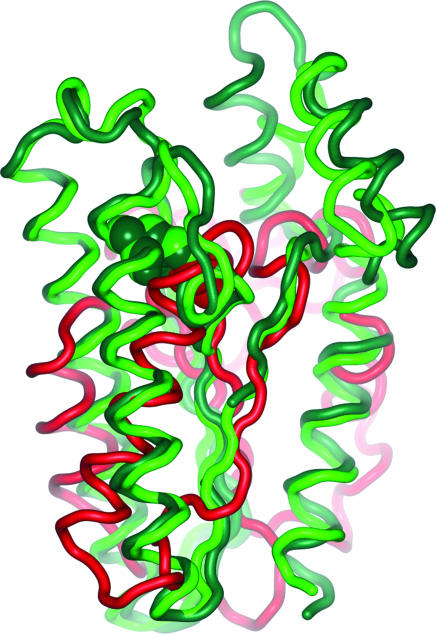
Fig. 4.
Superimposed catalytic cores of Cdc25A (red), 11βHSD1 (dark green), and 11βHSD2 (light green). Cdc25A and the pharmaceutically relevant 11βHSD1 exhibit an rms deviation of 4.13 Å at an alignment length of 80 residues. The sequence identities in this part amount to 5.0%. Also shown, in CPK representation, are the catalytic residues, Cys-430 (Cdc25A), Tyr-183 (11βHSD1), and Tyr-232 (11βHSD2). They share the same space although they derive from different locations when Cdc25A and 11βHSDs are compared.