FIG 1 .
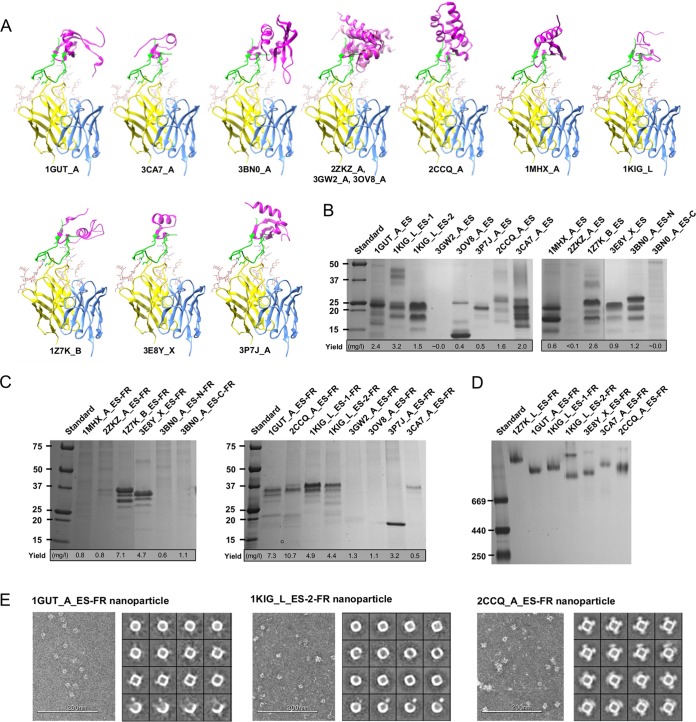
Expression of monomeric and particulate epitope scaffolds for the N332 supersite. (A) Twelve protein scaffolds identified by the scaffolding meta-server are superimposed onto the stems of a truncated V3 loop in complex with the broadly neutralizing antibody PGT128. All protein structures are shown as ribbon models, with the scaffold colored in magenta, epitope in green, PGT128 heavy chain in yellow, and light chain in cyan. The N301 and N332 glycans are shown as ball-and-stick models. Two scaffolds identified in the previous study (27), 1GUT_A and 3CA7_A, are included for comparison. The three structural homologs (2ZKZ_A, 3GW2_A, and 3OV8_A) are overlaid to facilitate structural comparison. (B) SDS-PAGE of 14 N332 scaffolds containing the truncated V3 loop under reducing conditions, with the estimated yield value indicated below the gel. (C) SDS-PAGE of 14 nanoparticles presenting the scaffolded N332 supersite under reducing conditions, with the estimated yield value indicated below the gel. (D) BN-PAGE of 7 expressed N332 nanoparticles. (E) Example micrographs and 2D class averages derived from negative-stain EM for three selected N332 nanoparticles, with a more complete EM analysis shown in Fig. S3. Design variants for scaffolds 3BN0_A and 1KIG_L were also included in the analysis whose results are shown in panels B to D.