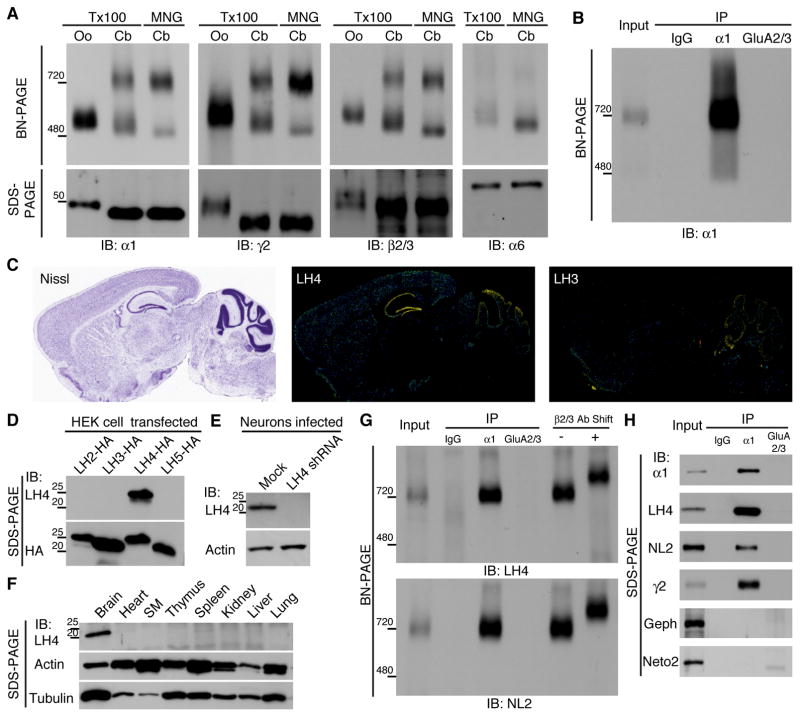
Figure 1. Native GABAAR complexes contain Lhfpl4 and neuroligin-2.
(A) Recombinant GABAAR expressed in Xenopus laevis oocytes (Oo) by injection of cRNAs of α1, β2 and γ2 GABAAR subunits migrated as a single band of 520 kDa using BN-PAGE when solubilized with Triton X-100 (Tx100). By contrast, the native GABAAR obtained from the cerebellum (Cb) migrated as two bands of 720 kDa and 500 kDa, respectively, with Tx100 solubilization. Using maltose-neopentyl glycol (MNG) solubilization, the native Cb GABAAR migrated as a strong band of 720 kDa and a weak band of 480 kDa. Immunoblots (IB) with antibodies against α1, γ2 and β2/3 subunits showed similar results, whereas the α6-containing Cb GABAAR migrates predominantly to 500 kDa and 480 kDa in Tx100 and MNG, respectively. (B) Immunopurified (IP) native GABAAR complexes obtained using an anti-α1 antibody from MNG-solubilized cerebella migrates to 720 kDa. A normal rabbit IgG and an anti-AMPA receptor GluA2/3 antibody were used as control. (C) The distribution of Lhfpl (LH) 4 and 3 mRNAs in mouse brain according to the Allen Brain Atlas (http://www.brain-map.org/). Nissl staining shows anatomy of the mouse brain (Nissl). LH4 is strongly expressed in hippocampus and cerebellum, whereas LH3 is expressed in cerebellum. (D) The antibody against LH4 recognized LH4 specifically, whereas the anti-HA antibody recognized all HA-tagged LH2/3/4/5 expressed in transfected HEK cells. (E) The anti-LH4 antibody recognized a band in lysate prepared from primary hippocampal neurons, which is eliminated in neurons treated with LH4 shRNA. (F) Among various mouse tissues, the anti-LH4 antibody recognized a 22 kDa band only in the brain. (G) Immunopurified α1-containing cerebellar GABAARs contain LH4 and neuroligin-2 (NL2). Pre-incubation of the immunopurified GABAARs with an anti-β2/3 antibody (Ab) increases the molecular weight. (H) On SDS-PAGE, LH4, NL2 and γ2 co-immunoprecipitated (IP) specifically with α1, but not with the AMPA receptor GluA2/3. Gephyrin (Geph) and Neto2 also failed to co-immunoprecipitate with α1.