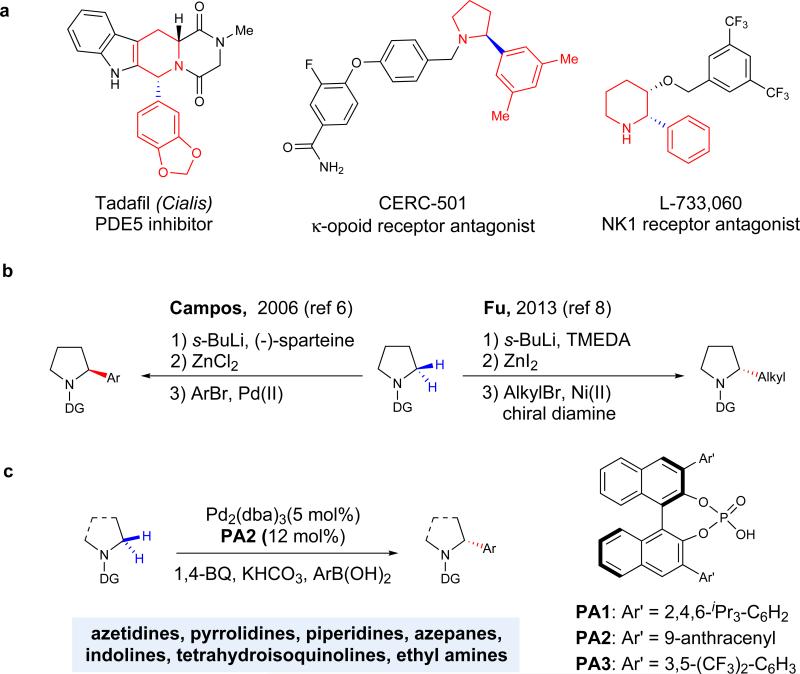
Figure 1. Examples of important chiral α-arylated cyclic amines and approaches for the construction of α-stereocenters.
a, Numerous drug molecules, such as Cialis, CERC-501, and L-733,060, contain non-racemic α-arylated cyclic amines. These structures are ubiquitous throughout biological systems, where the orientation of the α –substituent can have a dramatic effect on the activity of the resulting product. b, Traditional approaches for asymmetric arylation and alkylation of pyrrolidines proceed via α-lithiation to install aryl6 and alkyl8 substituents. While effective for pyrrolidine substrates, the procedures are far less effective for larger ring systems or those with sensitive functional groups. c, In this work, enantioselective α-C(sp3)–H coupling of amines is carried out in the presence of a chiral phosphate anion, which permits the arylation of a diverse array of aliphatic amines through the use of a thioamide directing group.