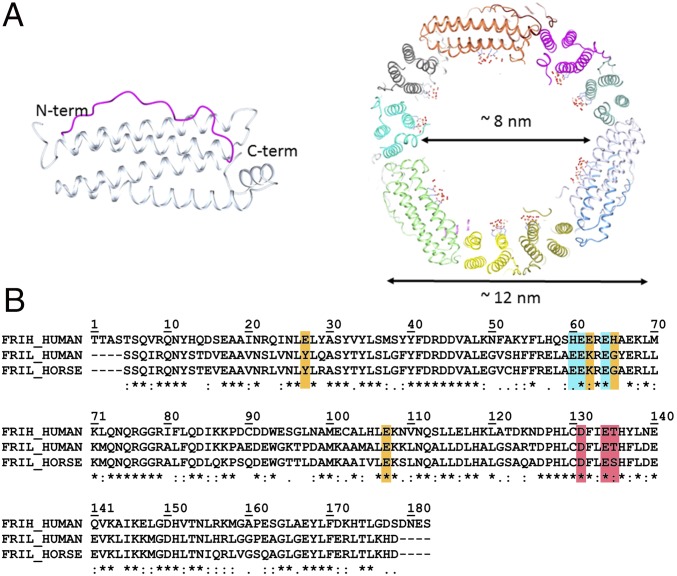
Fig. 1.
Ferritin main structural features and sequence alignment. (A Left) Schematic representation of the four-helix bundle subunit: Helices III and I are solvent-exposed and linked by a long loop (magenta); helices II and IV locate on the inner cage surface; a fifth short helix at the C terminus is tilted 60° with respect to the bundle axis. (Right) Section of the human L-ferritin (HuLf) cage. The trinuclear iron clusters sprout into the 8-nm cavity, surrounded by the protein shell of 12 nm external diameter. (B) Alignment of amino acid sequences of human H-ferritin (HuHf), HuLf, and horse spleen L-ferritin (HoLf) (FRIH_HUMAN, FRIL_HUMAN, and FRIL_HORSE, respectively), performed by Clustal Omega. All sequences are depleted of the initiator methionine. Shown in cyan are the residues binding the metal cluster in L-ferritin; in orange, the iron-binding amino acids in the ferroxidase site of the catalytically active H-subunit and the corresponding amino acids in the L-chains of HuLf and HoLf (both lacking the ferroxidase site); and, in magenta, the amino acids in the conserved pore responsible for iron entry.