Significance
Intrinsically photosensitive retinal ganglion cells (ipRGCs) respond to light via both rod/cone-driven synaptic input and intrinsic melanopsin-based phototransduction; however, these two pathways are not functionally redundant. Melanopsin-based phototransduction in ipRGCs is critical for the regulation of the pupillary light reflex (PLR), circadian light responses, masking, and sleep. Therefore, understanding the kinetics and shutoff mechanisms for the melanopsin-based photoresponse will reveal how it contributes to a myriad of behaviors that are controlled by ipRGCs. Here, we show that the melanopsin photoresponse shutoff due to C-terminal phosphorylation determines the kinetics of the intrinsic light response in ipRGCs, the PLR, and reentrainment, but not masking and phase angle of entrainment. These results highlight the elaborate control of how the melanopsin photoresponse regulates vastly different light-mediated behaviors.
Keywords: ipRGCs, photoentrainment, photoreceptors, phosphorylation, phototransduction
Abstract
Intrinsically photosensitive retinal ganglion cells (ipRGCs) express the photopigment melanopsin and mediate several non–image-forming visual functions, including circadian photoentrainment and the pupillary light reflex (PLR). ipRGCs act as autonomous photoreceptors via the intrinsic melanopsin-based phototransduction pathway and as a relay for rod/cone input via synaptically driven responses. Under low light intensities, where only synaptically driven rod/cone input activates ipRGCs, the duration of the ipRGC response will be determined by the termination kinetics of the rod/cone circuits. Little is known, however, about the termination kinetics of the intrinsic melanopsin-based phototransduction pathway and its contribution to several melanopsin-mediated behaviors. Here, we show that C-terminal phosphorylation of melanopsin determines the recovery kinetics of the intrinsic melanopsin-based photoresponse in ipRGCs, the duration of the PLR, and the speed of reentrainment. In contrast, circadian phase alignment and direct effects of light on activity (masking) are not influenced by C-terminal phosphorylation of melanopsin. Electrophysiological measurements demonstrate that expression of a virally encoded melanopsin lacking all C-terminal phosphorylation sites (C terminus phosphonull) leads to a prolonged intrinsic light response. In addition, mice expressing the C terminus phosphonull in ipRGCs reentrain faster to a delayed light/dark cycle compared with mice expressing virally encoded WT melanopsin; however, the phase angle of entrainment and masking were indistinguishable. Importantly, a sustained PLR in the phosphonull animals is only observed at brighter light intensities that activate melanopsin phototransduction, but not at dimmer light intensities that activate only the rod/cone pathway. Taken together, our results highlight how the kinetics of the melanopsin photoresponse differentially regulate distinct light-mediated behaviors.
In addition to detecting light for image-forming vision, the mammalian retina detects light for several non–image-forming visual functions, including photoentrainment of the circadian clock and regulation of pupil size, sleep, and mood. It has been shown recently that these non–image-forming visual functions require a subset of retinal ganglion cells that express the photopigment melanopsin (Opn4), a G protein-coupled receptor (GPCR) (1–3), and are known as intrinsically photosensitive retinal ganglion cells (ipRGCs) (4–6). Recent work has revealed a surprising diversity in ipRGCs, which are now known to comprise at least five distinct subtypes (M1–M5), based on molecular, morphological, and electrophysiological criteria and projection patterns (7). The ipRGCs are unique in that they can respond to light via two independent pathways: First, they can incorporate rod/cone input through classical retinal circuits; alternatively, they can respond intrinsically through melanopsin-based phototransduction machinery (8–12), which can extend many seconds beyond the actual light stimulus. Unlike the light responses of rods and cones, which show a rapid onset and decay on a subsecond time scale (5, 10, 13), the melanopsin-based intrinsic photoresponse of ipRGCs is relatively insensitive and sluggish, and can persist for tens of seconds up to minutes (5). The kinetics of the melanopsin-based ipRGC photoresponse are directly related to the duration of the phototransduction cascade. Surprisingly, little is known about the termination kinetics of the melanopsin photoresponse, and how these termination kinetics contribute to the multitude of melanopsin-mediated behaviors.
Melanopsin is unique compared with rhodopsin and cone opsins in that it has a long cytoplasmic tail that is predicted to have 38 serines and threonines that are potential phosphorylation sites by GPCR kinase (GRK) (14, 15). Recently, our in vitro studies have revealed that the C terminus phosphorylation is essential for the proper shutoff of the melanopsin light response (14, 15). Similar results have been reported using heterologous expression in Chinese hamster ovary cells and Xenopus oocytes (16). Additionally, we showed that light-dependent phosphorylation of melanopsin by GRK occurs in the mouse retina in vivo (14). Finally, a recent study revealed the role of melanopsin C-terminal phosphorylation on the kinetics of the electrical light response of ipRGCs, but its effects on non–image-forming behaviors remain to be tested (16).
Here, we show that eliminating all of the C-terminal putative phosphorylation sites results in sustained pupil constriction only following high light intensity stimulations. Consistent with the pupillary light reflex (PLR) results, we show that the electrical responses of ipRGCs persist for >15 min from single-cell recordings using patch-clamp electrophysiology. In addition, we find that the lack of GRK-mediated phosphorylation speeds up the reentrainment of animals to a shifted light/dark (L/D) environment but, surprisingly, does not affect masking or the phase angle of entrainment of activity rhythms. These studies suggest that ipRGC subtypes contributing to distinct non–image-forming functions may have different dependencies on GRK-mediated shutoff of the melanopsin photoresponse.
Results
Carboxyl-Terminal Serines and Threonines of Mouse Melanopsin Play a Crucial Role in PLR Kinetics Consistent with Electrophysiological Recordings.
Previously, we generated a mutant melanopsin [C-phosphonull (CPΦ); Fig. S1], in which all 38 putative C-terminal GRK phosphorylation sites (serines and threonines) were mutated to alanines and demonstrated that this mutant exhibits delayed deactivation kinetics using an in vitro calcium imaging assay (14, 15) (Fig. S2). A recent paper using multielectrode array (MEA) recordings showed that eliminating nine serines and threonines (S381, S384, T385, T388, S389, S391, S392, S394, and S395) in the C terminus of melanopsin, six of which were shown to be necessary for deactivation kinetics in our in vitro studies (T388, S389, S391, S392, S394, and S395), causes some ipRGCs to exhibit prolonged firing after cessation of the light stimulus (14–16). To investigate the in vivo importance of these phosphorylation sites for melanopsin-mediated behaviors, we expressed C terminus phosphonull melanopsin using adenoassociated virus serotype 2 (AAV2) encoding a Cre-dependent melanopsin and the fluorescent protein mRuby, which was introduced via bilateral intravitreal injections into Opn4Cre/Cre mice, a knock-in mouse line in which Cre recombinase replaces melanopsin at the Opn4 locus (7). Within 2 to 4 wk postinfection, mRuby fluorescence was readily visible in the soma and axons of a population of retinal ganglion cells, and the fluorescence was colocalized by immunofluorescence with virally encoded melanopsin (Fig. S3). Therefore, 4 wk after viral infection was chosen as a suitable time point for behavioral assays (17). The viral infection approach allowed us to compare melanopsin-mediated behaviors directly before and after viral infection in the same animal with or without melanopsin expression. Opn4Cre/Cre melanopsin knockout (MKO) mice, where the Cre recombinase replaced the melanopsin gene, exhibited a mean maximum consensual pupil constriction to 22.1% (±1.8 SEM) of the baseline pupil area in response to a high-intensity blue-light stimulus (∼log15 photons per cm−2⋅s−1) (Fig. 1 A and B). The PLR for Opn4Cre/Cre mice infected with AAV2 expressing WT melanopsin or C-terminal phosphonull showed no difference in response to the onset of light compared with the knockout animals (Fig. 1 A and B). After the cessation of the light stimulus however, the pupils of the Opn4Cre/Cre and Opn4Cre/Cre + AAV2 (WT melanopsin) mice dilated within seconds (Fig. 1B), whereas the pupils of the Opn4Cre/Cre + AAV2 (C terminus phosphonull) mice exhibited sustained constriction for the remainder of the 60-s recording (Fig. 1A and Fig. S4A). These results show that C-terminal phosphorylation plays an essential role in the termination of the melanopsin phototransduction pathway, allowing relatively rapid recovery of the dark state after a light stimulus.
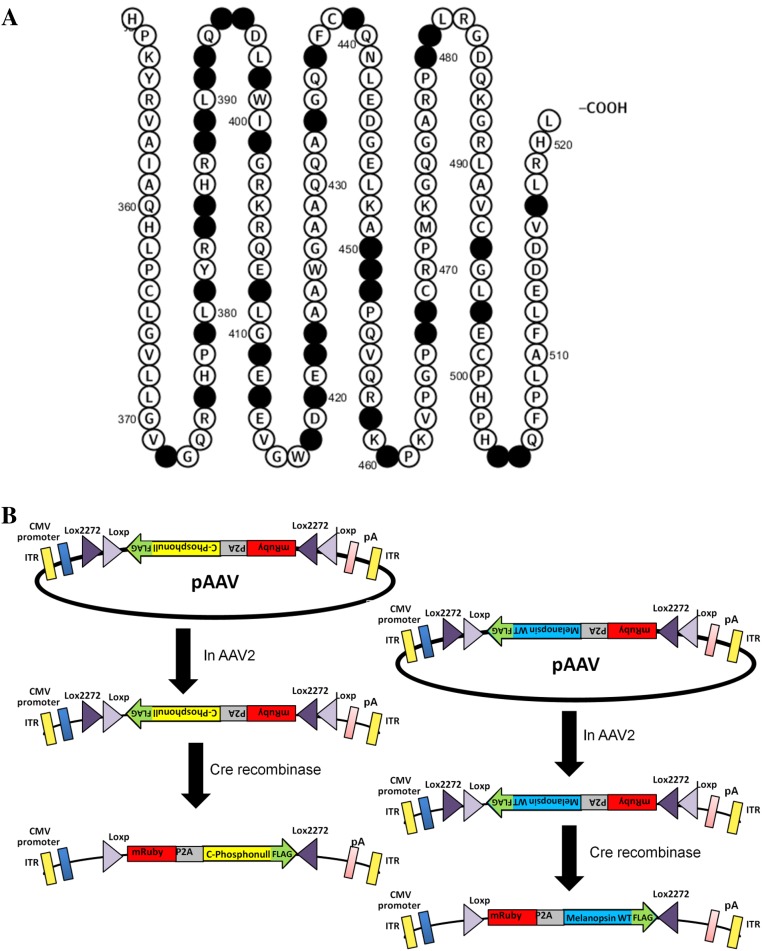
Fig. S1.
Melanopsin constructs used for the generation of the Cre-dependent cell type-specific viral expression system. (A) Graphic representation of the C terminus of CPΦ mouse melanopsin indicating all of the serines and threonines that were mutated to alanines (●), generated using Protter visualization platforms software. (B) Melanopsin WT and CPΦ constructs cloned into pAAV and critical features of pAAV required for viral packaging and for Cre-LoxP recombination and transduction. The first step indicates the final construct that gets packaged into AAV2 capsids, and the second step indicates the functional construct after Cre-LoxP recombination that is expected to be expressed in ipRGCs after viral transduction.
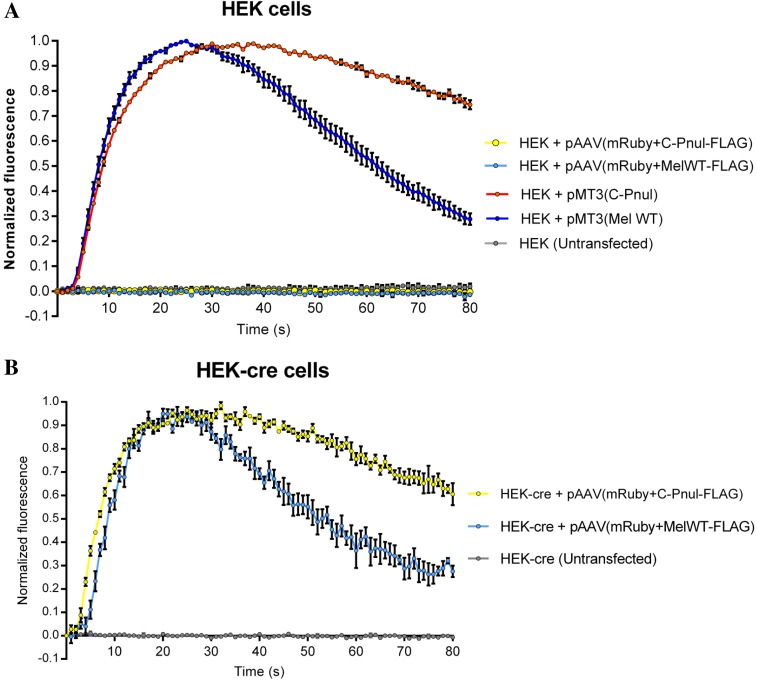
Fig. S2.
Expression and function of pAAV vectors with melanopsin WT (Mel WT) and CPΦ (C-Pnul) through Cre-LoxP recombination. Activity of melanopsin-FLAG and CPΦ-FLAG under the control of Cre-LoxP recombination is demonstrated using in vitro calcium imaging. The pAAV/(mRuby-P2A-melanopsin-FLAG) and pAAV/(mRuby-P2A-CPФ-FLAG) were transiently transfected into HEK293 (A) and HEK293-Cre (B) cells. As shown in A, pMT3 (melanopsin) and pMT3 (CPΦ) (mammalian expression vectors) were also transiently transfected into HEK293 cells. The pAAV/(mRuby-P2A-melanopsin-FLAG) and pAAV/(mRuby-P2A-CPФ-FLAG) expression in HEK293-Cre resulted in calcium response curves similar to the calcium response curves of pMT3 (melanopsin) and pMT3 (CPΦ) in HEK293 cells, indicating that the constructs are functional in the presence of Cre. However, pAAV/(mRuby-P2A-melanopsin-FLAG) and pAAV/(mRuby-P2A-CPФ-FLAG) expression in HEK293 resulted in calcium response curves that were flat, indicating that the constructs were nonfunctional in the absence of Cre.
Fig. S3.
Viral infectivity and colocalization of mRuby and melanopsin in the retina. Four weeks after intravitreal injection with either AAV2/(mRuby-P2A-melanopsin-FLAG) (A and C) or AAV2/(mRuby-P2A-CPΦ-melanopsin-FLAG) (B, D, and E), the retinas were harvested and immunostained with rabbit antimelanopsin antibody or imaged directly without immunostaining. Fluorescent images from five different retinas depict antimelanopsin staining on the plasma membrane of ipRGCs (A, B, D, and E) and mRuby in the cytoplasm (A–E). All infected cells in the field showed colocalization of mRuby and melanopsin in the same cells.
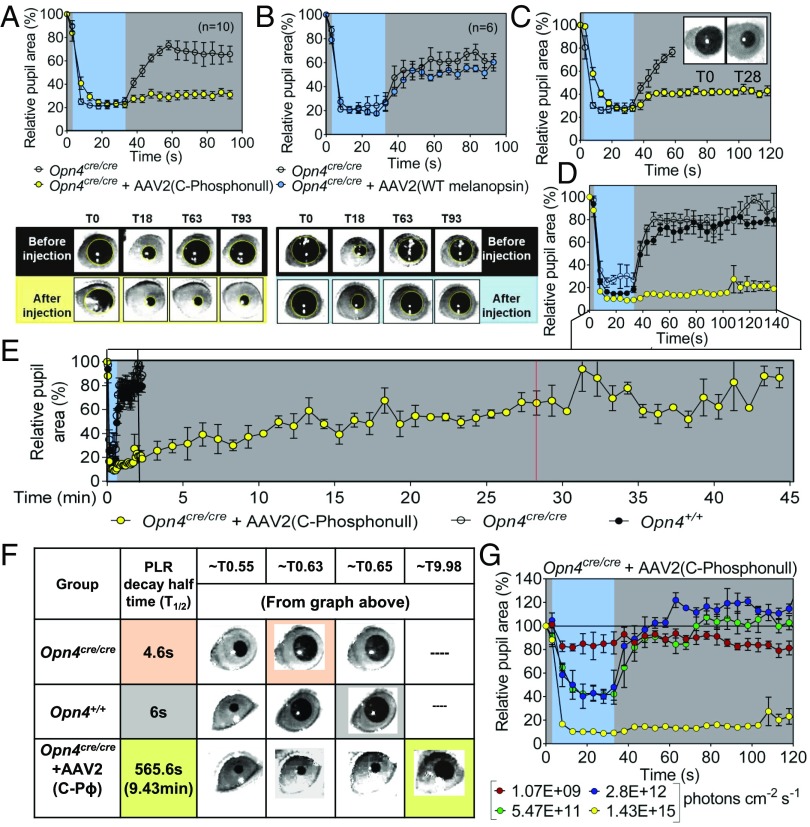
Fig. 1.
Expression of melanopsin phosphonull prolongs PLR. The relative pupil area of MKO (Opn4Cre/Cre), and MKO mice injected with AAV2 virus containing the C-terminal phosphonull melanopsin; AAV2 [mRuby-P2A-phosphonull (CPΦ)-FLAG, simplified to AAV2 (phosphonull)] (A, Top) and MKO, and MKO mice injected with WT melanopsin AAV2 [mRuby-P2A-melanopsin-FLAG; simplified to AAV2 (melanopsin)] (B, Top) is shown. Phosphonull-expressing mice (yellow circles; n = 10) exhibit prolonged PLR to a high-intensity blue light (∼1015 photons per cm−2⋅s−1), whereas WT melanopsin-expressing mice (blue circles; n = 6) are similar to MKO mice [open circles; n = 16 (n = 10 in A and n = 6 in B)]. (A and B, Bottom) Representative pupil images are shown, with T (time) in seconds. (C) Time course for pupil dilation in manually restrained mice after 30 s of blue light (∼1.4 × 1015 photons per cm−2⋅s−1): phosphonull (yellow circles, n = 4) and MKOs (open circles; n = 4). (D and E) Time course as in C in restrained mice using a head mount: phosphonull (yellow circles; n = 3), MKOs (open circles; n = 3), and WT (black solid circles; n = 3). The red line demarcates the point at which data are means from three animals; beyond the red line, data are from two animals. (F) Table showing t1/2 calculations for PLR decay. T0.55 corresponds to 33 s when the light is just turned off. (G) Intensity response curves for the phosphonull mice (n = 3). Statistical analyses conducted for before and after comparisons in A and B were linear mixed-effect models between MKO and AAV2 (melanopsin) (not significant, P = 0.209) and between MKO and AAV2 (phosphonull) (P ≤ 0.0001). Statistical analyses in D and E, using the Kruskal–Wallis test with Dunn’s multiple comparisons, were conducted between WT and AAV2 (phosphonull) (P ≤ 0.01) between MKO and AAV2 (phosphonull) (P ≤ 0.0001), and between WT and MKO (ns).
Fig. S4.
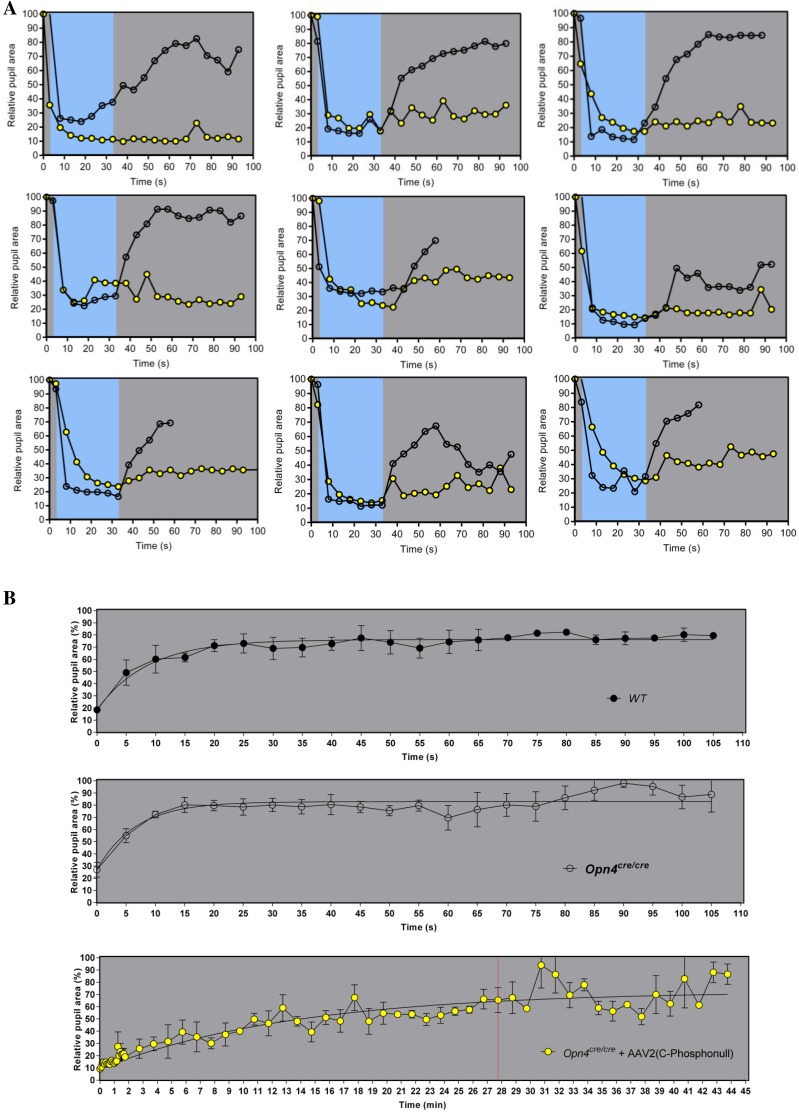
CPΦ animals show prolonged pupil constriction to high-intensity blue stimulus. (A) Individual PLR responses from nine animals before injection (Opn4cre/cre, open circles) and after injection [Opn4cre/cre + AAV2 (CPФ), yellow circles] demonstrate that individual animals show prolonged pupil constriction to a high-intensity blue-light stimulus. (B) Single exponential curve fits of the dilation phases of the PLR for Opn4+/+ (Top), Opn4cre/cre (Middle), and Opn4cre/cre + AAV2 (CPФ) (Bottom) for the extraction of PLR decay half-life (t1/2s).
In a recently published paper (16), the PLR was also measured in two animals that lack nine C-terminal serines and threonines, and the data suggested persistent pupil constriction up to 60 s after the light was terminated; however, no further time course information about pupil dilation was reported. To examine the full time course of pupil dilation in mice expressing the C terminus phosphonull melanopsin, we used a head-mount setup to record the PLR for up to 45 min. The maximum constriction of MKO animals was attenuated compared with WT animals as shown previously (18), whereas the C-terminal phosphonull animals showed a similar maximum response as observed in WT animals (Fig. 1D). Differences in these animals, however, were observed in the pupil dilation phase. Specifically, MKO mice and those mice expressing WT melanopsin exhibited a pupil dilation t1/2 of ∼6 s and 4.6 s, respectively (Fig. 1 E and F, Movies S1 and S2, and Fig. S4B). In contrast, the pupil of the C terminus phosphonull mice remained constricted 100-fold, with a dilation half-life of ∼9.4 min (Fig. 1 E and F, Movie S3, and Fig. S4B). This result reveals that C-terminal phosphorylation of mouse melanopsin is important for its deactivation on a time scale of seconds, and other deactivation mechanisms come into play at later times.
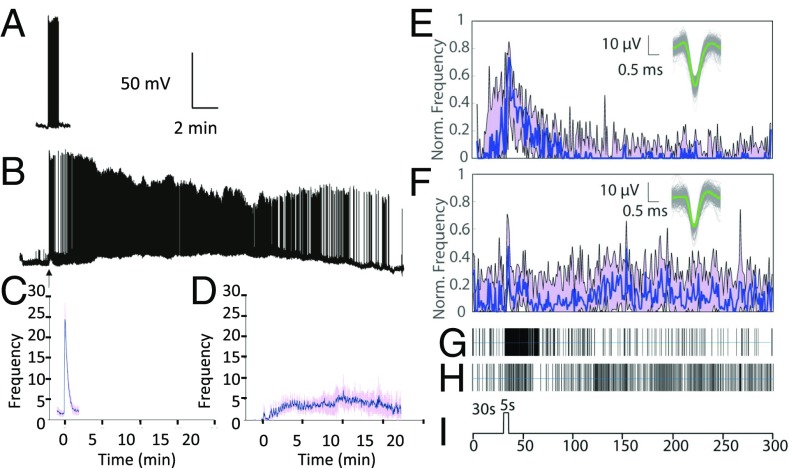
To determine if the longer constriction of the PLR is due to a prolonged light response in the ipRGCs, we carried out single-cell electrophysiology and MEA recordings from ipRGCs expressing virally encoded WT and phosphonull melanopsin. The sensitivity and duration of the WT melanopsin-based light response were consistent with light responses previously reported for endogenous melanopsin (6, 19). A 5-s stimulus of 480-nm light (1 × 1015 photons per cm−1⋅s−1) elicited a light-dependent increase in spiking activity (up to 25 Hz) in 18 of 21 mRuby-expressing cells, which typically terminated in <1 min (t1/2 = 22 s postpeak, with 90% recovery in 52 s) (Fig. 2 A and B). In contrast, light responses were much more difficult to detect in ipRGCs expressing the melanopsin C terminus phosphonull. In all, only four of >50 cells that were recorded exhibited a detectable intrinsic light response. The light response in these cells, however, was dramatically prolonged compared with the cells expressing WT melanopsin, showing robust spiking activity persisting for at least for 15 min after the cessation of the light stimulus (Fig. 2 A and B). In a single cell that remained viable for an extended duration, the firing rate eventually returned to near baseline after more than 20 min. For all of these cells, the light response onset was more sluggish than seen in ipRGCs expressing WT melanopsin, often taking >5 min to reach the peak firing rate (Fig. 2B). The peak firing rate was also depressed compared with the peak firing rate of ipRGCs expressing WT melanopsin (Fig. 2B). To increase our chances of detecting light-responsive retinal ganglion cells with the phosphonull melanopsin, we performed MEA recordings on retinas expressing either WT or phosphonull melanopsin. In the MEA recordings, ipRGCs expressing WT and phosphonull melanopsin were detected in approximately equal numbers (about 5% of all identified cells), and, typically, several ipRGCs were detected in each retinal preparation. We found that for the MEA recordings from WT animals, the majority of ipRGCs shut off within 50 s after the light stimulation. In contrast, for the phosphonull animals, ipRGC responses persist for at least 4.5 min, which is the longest recording time (Fig. 2C). Together, the single-cell data and the MEA recordings show that the persistent light response in ipRGCs is consistent with the persistence response of the PLR in darkness.
Fig. 2.
Phosphonull melanopsin-expressing ipRGCs show a prolonged light response. (A–D) Current-clamp recordings from ipRGCs expressing WT (A) and phosphonull melanopsin (B). Cells were initially maintained at −60 mV. Light stimulation (arrow; 480 nm, 5 s, 1 × 1015 photons per cm−2⋅s−1) increased action potential frequency, which terminated within 1 min in WT but was prolonged (>20 min) for phosphonull melanopsin. Spike frequency (averaged over 2-s bins) is shown as a function of time in ipRGCs expressing WT (C; n = 10) and phosphonull (D; n = 4); error bars are SEM. (E–I) MEA recordings of retinas from WT or AAV2 (phosphonull). Normalized frequency response curves to a 5-s blue-light pulse (digitized in I) of nine (E, WT) and 11 (F, phosphonull) ipRGCs are shown. Data (binned to 1-s bins and normalized to the maximum firing frequency) are represented as the median (blue curve) and 25–75% interquartile interval (purple area). Maximum frequency ranged from 8 to 39 Hz for WT and from 10–48 Hz for ipRGCs. WT ipRGCs return to baseline at about 60 s after the 5-s light pulse, whereas phosphonull ipRGCs continue to fire at a reduced and inconsistent rate for the whole length of this recording (4 min and 25 s after the light is turned off). Example spike raster plots for individual light responsive units for WT (G) and phosphonull (H) ipRGCs (n = 4 for WT and n = 3 for phosphonull) are shown. Registered, overlaid spikes (gray curves) for the individual cells in G and H are shown in E and F (Insets), together with their respective average spikes (green line). (I) Digital synchronized signal shows the experimental protocol.
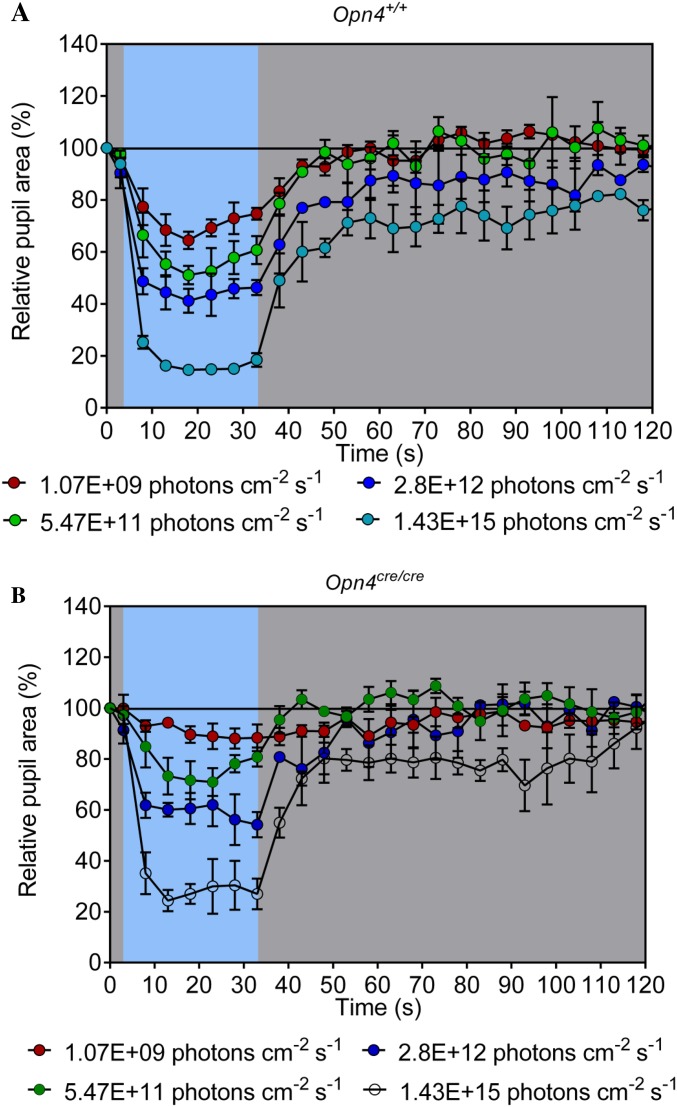
To determine if the longer pupil dilation times are specific to melanopsin-based phototransduction and not to rod/cone input (18, 20), we measured the PLR at four different light intensities to generate intensity response curves. As expected, the dilation phase of the PLR did not show any difference between Opn4Cre/Cre + AAV2 (C terminus phosphonull) and Opn4Cre/Cre at low light intensities, where the PLR is driven by rod/cone input (18, 20) (Fig. 1G and Fig. S5). At intensities >15 log (photons per cm−2⋅s−1), however, which is known to activate melanopsin-based phototransduction (18, 19), the curve for Opn4Cre/Cre + AAV2 (C terminus phosphonull) deviated from Opn4Cre/Cre and WT animals (Fig. 1G and Fig. S5). This result demonstrates that GRK-based shutoff of melanopsin-based phototransduction through C-terminal phosphorylation is important for the proper kinetic regulation of the PLR.
Fig. S5.
Opn4cre/cre (MKO) and Opn4+/+ (WT) animals do not exhibit the CPΦ characteristic phenotype at high light intensity. Intensity response curves of PLR for Opn4+/+ (A) and Opn4cre/cre (B) at four different blue light intensities are shown.
The Delayed Shutoff Properties of the C Terminus Melanopsin Phosphonull Mutant Renders Phase Delays More Efficient but, Surprisingly, Does Not Affect the Phase Angle of Entrainment.
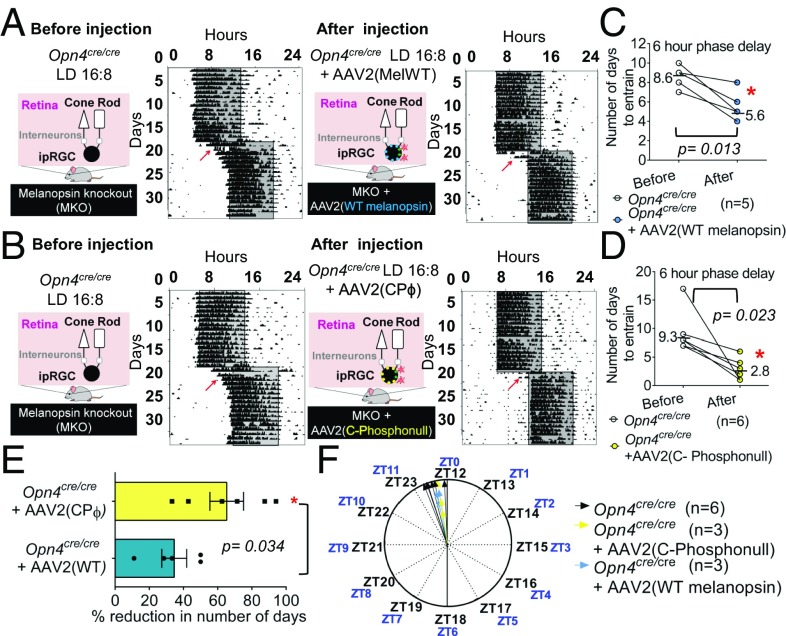
To determine if C-terminal phosphorylation of melanopsin plays a role in circadian photoentrainment, we placed animals under a 16-h/8-h (16:8) L/D cycle before and after injections with AAV2 (WT melanopsin) or AAV2 (C terminus phosphonull). Opn4Cre/Cre mice were able to photoentrain to 16:8 L/D due to the presence of intact rods and cones (Fig. 3A, Top and B, Top), and Opn4Cre/Cre mice infected with WT melanopsin also showed photoentrainment as expected (Fig. 3A, Bottom). Based on our PLR data, we expected that exposing the Opn4Cre/Cre mice infected with C terminus phosphonull melanopsin to 16 h of light would cause these animals to have a severely delayed phase angle of entrainment, because only 5 s of light stimulation leads to more than 15 min of melanopsin-based spiking activity. Surprisingly, we failed to see any changes in the phase angle of entrainment in these animals (Fig. 3F). This result indicates that after prolonged light stimulation, shutoff mechanisms independent of GRK-mediated C-terminal phosphorylation contribute to melanopsin phototransduction termination even in the presence of light input.
Fig. 3.
Phosphonull and WT melanopsin rescue the jet-lag deficit observed in MKO mice. (A) Graphical depiction and representative actograms using a paradigm of a 16:8 L/D cycle of one MKO before (Top) and after (Bottom) injection with AAV2 (melanopsin; red particles are virus). MelWT, melanopsin WT. (B) Same as in A, but now with AAV2 (phosphonull). Red arrows indicate the 6-h jet lag. (C) Number of days for reentrainment: MKO before and after injection with AAV2 (phosphonull; n = 6). (D) Number of days for reentrainment with AAV2 (melanopsin). (E) Percent reduction in number of days taken by MKOs to reentrain after injection with either AAV2 (melanopsin; blue) or AAV2 (phosphonull; yellow). (F) Comparison of phase angle of entrainment for MKO (black, n = 6), AAV2 (melanopsin; blue, n = 3) and AAV2 (phosphonull; yellow, n = 3). Statistical analyses conducted in C and D for before and after comparisons were paired two-tailed t tests comparing MKO and AAV2 (melanopsin; P = 0.013) and MKO and AAV2 (phosphonull; P = 0.023) (*P ≤ 0.05). The comparison between AAV2 (melanopsin) and AAV2 (phosphonull) in E was conducted by an unpaired t test with Welch’s correction test (P = 0.034) (*P ≤ 0.05). Statistical analyses for F were paired two-tailed t tests comparing MKO and AAV2 [melanopsin; not significant (ns), P = 0.388] and MKO AAV2 (phosphonull; ns, P = 0.47). A comparison between the three groups was conducted by the Kruskal–Wallis test (ns, P = 0.150).
To investigate the ability of the animals to reentrain to a shifted L/D cycle, we delayed the dark onset by 6 h, mimicking a transmeridian flight from Amsterdam to New York, in Opn4Cre/Cre mice to 16:8 L/D before and after injections with AAV2 (WT melanopsin) and AAV2 (C terminus phosphonull). Before viral injections, Opn4Cre/Cre mice took an average of 9 d (±0.85 SEM) to shift their activities and entrain to the delayed L/D cycle (Fig. 3 A and B, Top and C and D, Left). Opn4Cre/Cre + AAV2 (WT melanopsin), on average, needed 5.6 d (±0.68 SEM) to shift their activities (Fig. 3A, Bottom and C, Right), whereas Opn4Cre/Cre + AAV2 (C terminus phosphonull) needed only an average of 2.8 d (±0.79 SEM) (Fig. 3B, Bottom and D, Right). The days needed to photoentrain for the infected animals were significantly different from the days needed for their uninfected counterparts (Fig. 3 C and D). The presence of melanopsin (WT or C terminus phosphonull) renders Opn4Cre/Cre mice more efficient in reentraining to phase delays (Fig. 3 A and B).
Opn4Cre/Cre + AAV2 (C terminus phosphonull) mice exhibited a 65.3% (±9.8 SEM) reduction in the number of days taken to reentrain in comparison to Opn4Cre/Cre mice (Fig. 3E). Similarly, Opn4Cre/Cre + AAV2 (WT melanopsin) mice exhibited a 34.6% (±7.3 SEM) reduction in the number of days taken to reentrain in comparison to Opn4Cre/Cre mice (Fig. 3E). The reduction of days needed for reentrainment in the phosphonull mice was significantly different from WT melanopsin mice.
Both WT and C Terminus Phosphonull Melanopsin Rescue Negative Masking Deficit in MKO.
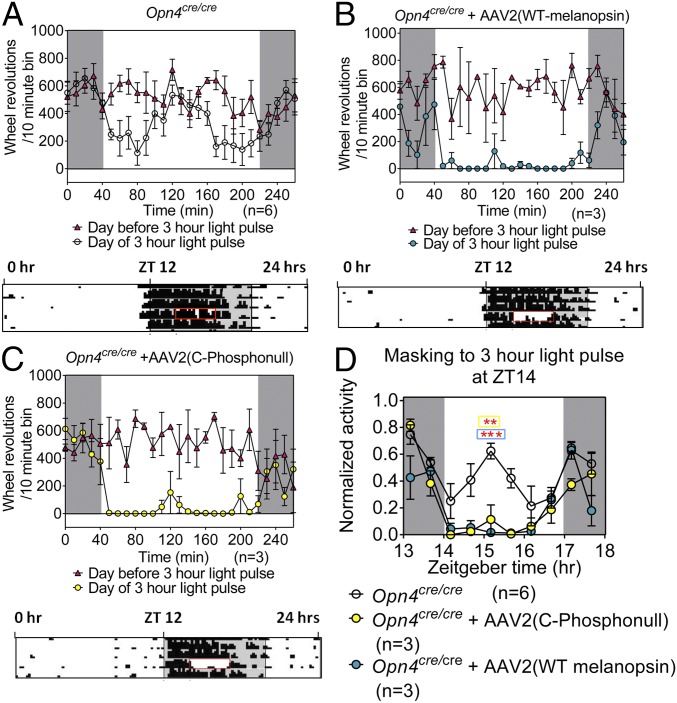
In addition to circadian photoentrainment, masking is another mechanism by which animals exhibit light-mediated changes in behavior (21). Nocturnal animals reduce their activity during their subjective night when exposed to a high-intensity light stimulus. MKO animals exhibit a deficit in negative masking at high light intensities (21) (Fig. 4A). Opn4Cre/Cre mice were housed in a 16:8 L/D cycle and given a 3-h light pulse (500 lux) at Zeitgeber time 14 (ZT14) (2 h after the onset of darkness). When Opn4Cre/Cre mice were injected with either AAV2 (WT melanopsin) or AAV2 (C terminus phosphonull), a rescue of this deficit was observed (Fig. 4 B and C). There was no apparent difference in the persistence of the masking response after the cessation of the light stimulus between either melanopsin construct (Fig. 4D). In fact, similar to the phase angle of entrainment, the masking response is similar between melanopsin and C terminus phosphonull (Figs. 3F and 4D).
Fig. 4.
Both WT and phosphonull melanopsin rescue the masking deficit in MKO. A comparison of wheel-running activities recorded in 10-min bouts between the day before and day of a 3-h light pulse in MKO (A), AAV2 (melanopsin) (B), and AAV2 (phosphonull) (C) is shown. A 3-h light pulse initiated at Zeitgeber time 14 (ZT14) is indicated by the red box. (D) Comparison of the normalized activities of the three groups represented in 20-min bins from ZT13 to ZT18. Wheel revolutions were normalized to the highest bout, which occurs outside of the window of time shown here. Statistical analyses conducted in A–C for before and after comparisons were linear mixed-effect models comparing activities on the day before and day of a 3-h light pulse in MKO (P = 0.042), AAV2 (melanopsin; P = 0.009), and AAV2 (phosphonull; P = 0.045). Comparison of the three groups in D was conducted by two-way ANOVA with multiple comparisons (**P ≤ 0.01, ***P ≤ 0.001).
Discussion
We used AAV2 viral expression of melanopsin and the C terminus phosphonull form of melanopsin to study the effect of restoration of intrinsic light responses on MKO animals at adult stages. GPCRs commonly undergo deactivation/desensitization through light-dependent phosphorylation of multiple C-terminal serine and threonine residues by GRKs, and subsequent arrestin-mediated termination of receptor signaling (22). Studies on rhodopsin indicate that multiple phosphorylations in the C terminus are required for complete quenching of the rhodopsin-mediated light response in rod photoreceptors (23). Surprisingly, the significance of melanopsin’s C terminus phosphorylation in ipRGC function has been a subject of debate in the field (24–26). This controversy arose from the observation that the shutoff of melanopsin phototransduction is prolonged and variable after the cessation of light stimulation. A recent paper in which the authors used MEA recordings from retinas infected with melanopsin lacking the six serines and threonines (T388, S389, S391, S392, S394, and S395) that we reported earlier (14, 15), plus three additional serines and threonines (S381, S384, and T385), showed that melanopsin deactivation is important in vivo (16). In this study, we determine the role of C terminus phosphorylation of melanopsin for several light-dependent behaviors and demonstrate that C-terminal phosphorylation is essential for the regulation of PLR and phase delay, but not masking behavior or the phase angle of entrainment.
We found that even in the absence of all of the C terminus phosphorylation sites, the PLR eventually returns to baseline in minutes. This finding suggests that alternative shutoff mechanisms are involved in terminating the melanopsin phototransduction pathway. The slow termination responses of phosphonull melanopsin mutant-expressing ipRGCs, however, did not affect the phase angle of entrainment, but did cause faster reentrainment in response to a jet-lag paradigm. These results highlight the exciting finding that the C terminus contribution to melanopsin function is dependent on both behavior and light duration.
Although C-terminal phosphorylation sites are crucial for normal deactivation of melanopsin, we do not know precisely the candidate GRK involved in this function. GRK1–GRK6 are expressed in the retina, and GRK2, GRK3, and GRK5 are known to be expressed in ipRGCs (14). We demonstrated that GRK2 and GRK3 are coimmunoprecipitated with melanopsin in ipRGCs in a light-dependent manner (14). Despite this evidence that suggests an interaction between GRKs and melanopsin, it has been shown that GRK2 regulates melanopsin activity only minimally, and only in very young animals (25). It was demonstrated that genetic deletion of GRK2 does not significantly impact melanopsin activity or ipRGC function in adult animals (25). We propose that the lack of phenotype in adult animals could be explained by the presence of other GRKs in ipRGCs (25). In the absence of GRK2, melanopsin could be phosphorylated by GRK3, thereby compensating for the loss of GRK2. Further studies that completely ablate all GRKs in a conditional manner may shed light on the identity of the GRK primarily phosphorylating melanopsin in native conditions. Melanopsin is a promiscuous GPCR in terms of its interaction partners; it associates with more than one type of G protein (27–29). Similarly, it is likely that melanopsin is nonselective in the association of GRK type.
Our PLR data are consistent with both single-cell and MEA recordings. We observed, however, that for single-cell recordings, very few cells were light-responsive in the C terminus phosphonull retinas. We suggest that the lack of responsivity is caused by exposure to 540-nm light, which is required to locate virally infected cells expressing mRuby. This 540-nm light is capable of activating melanopsin phototransduction, which we suspect causes the light response of the ipRGCs to become saturated. Consistent with this explanation, we observed that ipRGCs from WT-infected animals had a resting membrane potential of −54 mV (±7.5 SEM), whereas ipRGCs from phosphonull-infected animals had a resting membrane potential of −46 mV (±3.5 SEM). Furthermore, when we used MEA recordings, in which ipRGCs do not need to be located and retinas are prepared in darkness, we were able to detect more light-responsive ipRGCs in the C terminus phosphonull retinas. However, similar to single-cell recordings, the ipRGCs in these C terminus phosphonull retinas showed reduced responses to light onset. One possibility is that prolonged signaling of the C-terminal phosphonull melanopsin may result in down-regulation of components of the phototransduction cascade.
How can we reconcile the differences in the contribution and duration of the C terminus phosphorylation to the variety of the melanopsin-based behavioral light responses? The most logical possibility is that different ipRGC subtypes (7) use distinct shutoff mechanisms due to differential expression of GRKs, for example. Because ipRGC subtypes contribute to different behaviors (4, 6, 7, 21), the importance of the shutoff response is going to depend on which subtype is driving a specific behavior. Another possibility is pigment regeneration in the dark after light exposure. For example, in the PLR, after the light is turned off, the pupil eventually fully dilates to baseline levels. This finding indicates that a pathway independent of C terminus phosphorylation can function in the dark to restore melanopsin close to the basal state. Such a pathway could include the dissociation of the chromophore from melanopsin, followed by regeneration of the inactive form due to the rebinding of the cis form of the chromophore. This explanation is consistent with the idea that melanopsin seems to return to the basal state under dark conditions (30). It is important to highlight that a similar phenomenon is observed in rod photoreceptors lacking rhodopsin kinase, where although the light response is prolonged, it does deactivate using an alternate deactivation mechanism described by a single exponential function and a time constant of 3 s (31).
A surprising outcome of this study is that in CPϕ animals, even after 16 h of light stimulation, the phase angle of entrainment was similar and masking responses were indistinguishable from masking responses in animals expressing WT melanopsin. What are the possible mechanisms for such an effect? It is hard to come up with a totally satisfying answer to this question; however, we advance three different possibilities: (i) Melanopsin is an R-type opsin and is either a bistable or tristable visual pigment; therefore, under continuous light stimulation, photoisomerization could revert some active melanopsin back to the basal state (16, 26); (ii) different ipRGC subtypes use distinct shutoff mechanisms for the ipRGC responses, with some subtypes using a GRK-independent pathway; and (iii) under prolonged light stimulations, shutoff mechanisms involving components of the phototransduction pathway downstream of the melanopsin pigment are involved in terminating the response. Regardless of the reason for these differences, our study has just scratched the surface for the complexity of the shutoff responses in ipRGCs. In addition, we have demonstrated that light-activated phosphorylation of melanopsin’s C terminus is an important posttranslational modification regulating the lifetime of active melanopsin for regulating the PLR and the speed of reentrainment.
Methods
Physiological and Behavioral Analyses of Animals.
All experiments were conducted in accordance with NIH guidelines and were approved by the institutional care and use committee of the universities involved. All protocols were in accordance with The Johns Hopkins University Animal Care and Use Committee guidelines. All procedures for patch-clamp recordings were carried out in compliance with Washington State University’s Institutional Animal Care and Use Committee guidelines. We used physiological tests to determine the photoresponse of ipRGCs using MEA recordings (30–34)* and single-cell patch-clamp recordings (35). We used behavioral tests to determine the wheel-running activity of the animals (36, 37), PLR (18, 20), phase shifting and phase angle of entrainment (36, 37), and direct effects of light on activity (21).
SI Methods
Animals.
All animals used for behavior were Opn4Cre/Cre mice on a mixed BL/6;129SvJ background and were between 1 and 2 mo of age at the start of the behavioral experiments. All protocols were in accordance with The Johns Hopkins University Animal Care and Use Committee guidelines. Animals were individually housed in plastic cages with steel wheels and were kept in light-tight boxes on an L/D protocol of 16 h of light and 8 h of dark (16:8 L/D) with 500-lux (lx) overhead white light. Food and water were made available ad libitum. Behavioral experiments were initially conducted for ∼30 d, followed by viral injection, 4 wk of postinjection recovery for expression of virally encoded proteins (17), and ∼30 d of postinjection behavioral studies. Animals were euthanized after postinjection behavioral studies to obtain retinas for immunofluorescent verification of melanopsin expression.
Virus.
AAV2 (mRuby-P2A-melanopsin-FLAG) with a viral titer of 5 × 1012 vector genomes (vg)/mL and AAV2 (mRuby-P2A-C terminus phosphonull-FLAG) with a viral titer of 2.5 × 1012 vg/mL were prepared by the Z.W. laboratory (17).
Pupillometry.
All animals used for PLR were dark-adapted for 1 h before the experiment. Animals were manually restrained, and the left eye was exposed to a 30-s stimulus of high-intensity blue light using an LED bulb (Superbright LEDs). Light intensity was attenuated using neutral density filters (474 nm; intensities used were ∼1 × 109, ∼5.5 × 1011, ∼2.8 × 1012, ∼1 × 1015, and ∼1.4 × 1015 photons per cm−2⋅s−1). The consensual PLR was recorded from the contralateral eye using a SONY Handycam (DCR-HC96). A second type of PLR recording was conducted by restraining the mice using a head post as described by Cahill and Nathans (32). Video recordings were analyzed by generating screen shots of the video in Windows Media Player every 5 s. In the head-mount PLR experiment, screen shots were generated every 5 s for the first ∼2 min of the video recordings for CPϕ and every 1 min for the remainder of the recordings. The pupil area was measured using the circular selection and measure tools in ImageJ software (NIH). PLR recordings and measurements were conducted identically for pre- and postinjection measurements.
PLR decay half-life was calculated by fitting a single exponential curve to the dilation phase of the PLR data from the head-mount restraint PLR experiments and extracting half-life (t1/2s) using the in-built function in GraphPad Prism 6 software (Fig. S4). The Yo value (y-axis initial value) was constrained to the mean pupil size in each group at T33 (time of light off), and the K-value was constrained to >0.
PLR Video Recordings.
PLR videos were obtained as described in Methods (pupillometry) using the head-mount restraint method. The videos were loaded into Windows Movie Maker and converted to grayscale. The videos were then sped up eightfold, captions were added, and the videos were converted to the Audio Video Interleaved (AVI) format.
Intravitreal Viral Injections.
Mice were anesthetized by i.p. injection with 0.5 mL of avertin (20 mg/mL; volume was adjusted based on body weight of the mice). One microliter of AAV2 (mRuby-P2A-melanopsin-FLAG) or AAV2 (mRuby-P2A-C terminus phosphonull-FLAG) was dispensed onto a strip of parafilm. The viral preparation was then drawn up into a micropipette (P0674; Sigma) connected to the hose of a picoinjector (model PLI-90; Harvard Apparatus), which, in turn, was connected to a compressed nitrogen gas tank. Injection pressure was maintained at 30 psi using a regulator. The anesthetized animal was placed under a dissection microscope, and forceps were used to open the eyelids and expose the eye. The eye was punctured by the injection needle near the ora serrata, aiming the tip toward the center of the eyeball. Depression of the foot switch created a surge of pressure to deliver the viral preparation from the microcapillary tube into the vitreous. After injection, the animals were kept warm to allow them to recover from the injection and wake up. They were then returned to their cages in the same setting.
Wheel-Running Activity.
Animals were housed singly in a 16:8 L/D (500-lx white) cycle in cages with 4.5-inch steel running wheels. The wheel-running activity was continuously monitored in 10-min bins using VitalView software (Mini Mitter). The L/D cycle was controlled using a timer connected to the light-tight box in which the cages were kept.
Jet-Lag Paradigm.
Opn4Cre/Cre mice were kept in the 16:8 L/D cycle for ∼2 wk, followed by a 6-h phase delay, mimicking the jet lag experienced in a western-bound trans-Atlantic flight. The animals were allowed to photoentrain to this shifted light cycle. The average activity offset of the animals during the stable part of their actograms (after phase delay) was calculated. The number of days taken to reach that average offset was calculated, and this calculation yielded the value for the number of days required to entrain to phase delay. This procedure was repeated in the same mice 4 wk after viral injection for comparison, and a before and after comparison analysis was drawn. The percent reduction in the number of days taken to shift activity to the delayed light cycle was calculated as follows:
Masking.
The Opn4Cre/Cre mice kept in a 16:8 L/D cycle after the phase delay analysis were subjected to a 3-h light pulse (500-lx white) 2 h after the onset of subjective night (ZT14) on a single specific day. The wheel-running activities recorded in 10-min bouts on the day before and day of the 3-h light pulse were calculated. The normalized activity before, during, and after this 3-h light pulse was calculated over a time window of 5 h (ZT13 to ZT18). This experiment was repeated in the same mice 4 wk after the viral injection, and a before and after comparison analysis was made.
Phase Angle of Entrainment.
The average onset of activity of each group of animals was measured over a period of 10 d in the stable part of their actograms. The deviation of average onset from the time of dark onset was calculated and plotted for each animal in a 12-h clock format.
Immunofluorescence.
Mice were euthanized by cervical dislocation after i.p. injection with 1 mL of avertin (20 mg/mL). The eyes were removed and initially fixed whole in 4% (vol/vol) paraformaldehyde for 30 min. The cornea was then removed, and the eyecup with the exposed retina was fixed in 4% paraformaldehyde for an additional hour. The eyecups were washed three times with 1×PBS for each of the wash steps before application of blocking buffer [0.3% Triton, 4% (vol/vol) goat serum], primary antibody, and secondary antibody. Immunofluorescence was performed using rabbit antimelanopsin (1:1,000, AB-N38; Advanced Targeting Systems) as the primary antibody with a 2-d incubation period, followed by goat anti-rabbit IgG 488 (1:1,000, A11008; Life Technologies) as the secondary antibody. After immunostaining, the retinas were mounted and imaged using a Zeiss Axioimager M1 microscope. The images were processed in ImageJ.
Electrophysiology.
Methods for patch-clamp recordings from ipRGCs were similar to the methods previously described. All procedures were carried out in compliance with Washington State University’s Institutional Animal Care and Use Committee guidelines. Following intravitreal injection of AAV2 encoding either WT or phosphonull melanopsin, male and female Opn4Cre/Cre mice were individually housed on a 12:12 L/D cycle with ad libitum access to food and water, allowing 2–6 wk for AAV-mediated melanopsin expression. Before recording, mice were dark-adapted overnight and killed by cervical dislocation following isoflurane anesthesia; the eyes were enucleated, rinsed in PBS, and hemisected at the ora serrata. The retinas were extracted under dim white light (3 × 10−5 W/cm2) in oxygenated, bicarbonate-buffered Ames media (US Biologicals); cut into quarters; mounted on a nitrocellulose filter with a hole providing access to the retinal ganglion cell layer; and stored in the same solution in the dark at room temperature. Retinas could be maintained for more than 4 h under these conditions. In an attempt to enhance the frequency of light responses, a subset of retinas expressing phosphonull melanopsin were prepared under dim red light and then stored in Ames media supplemented with 40 μM 9-cis retinal (Sigma).
Patch-Clamp Recordings.
Whole-mounted retinas were transferred to the recording chamber, where they were maintained in “bubbled” Ames media (35 °C) flowing at 5 mL⋅min−1. Retinal ganglion cells were visualized with infrared Dodt gradient contrast optics through an inverted Olympus microscope (BX51WI) using a Photometrics Cool Snap ES2 camera and NIS Elements Advanced Research imaging software (v3.00; Nikon). The microscope was mounted on a Sutter X-Y translations stage; electrode positioning was accomplished via a micromanipulator (ROE-200; Sutter). Micropipettes were fabricated from filamented borosilicate glass with resistances of 5–9 MΩ when filled with a potassium-based internal solution containing 125 mM KMeSO4, 2 mM CaCl2, 2 mM MgCl2, 10 mM EGTA, 10 mM K-Hepes, 0.5 mM NaGTP, and 2 mM MgATP (pH 7.2). Synaptic blockers, which allowed for isolation of intrinsic melanopsin-based light responses, were added to the Ames media from DMSO stocks to the following concentrations: 3 mM kynurenic acid (Sigma), 30 μM 6,7-dinitroquinoxaline-2,3-dione (DNQX) (Tocris), 50 μM DL-AP4 (Tocris), 10 μM (+)-bicuculline (Sigma), and 3 μM strychnine (Sigma). Virally infected retinal ganglion cells were identified by mRuby fluorescence using a minimal exposure to 540-nm light (<1 s; 1 × 1014 photons per s−1⋅cm−2; Lambda DG-4, Sutter Instruments). Current-clamp recordings were obtained in the whole-cell configuration using a HEKA EPC-10 amplifier and PatchMaster software. Recordings were filtered at 3 kHz, and samples were filtered at 10 kHz. Melanopsin-based light responses were elicited with a 5-s blue-light stimulus delivered from a Lambda DG-4 light box (Sutter Instruments); intensities ranged from dimmest (1.0 × 1013 photons per s−1⋅cm−2) to 2 × 1015 photons per s−1⋅cm−2. The intensity of background illumination from the computer monitors throughout these experiments was no greater than 1 × 10−9 W/cm2; for later experiments with retinas expressing phosphonull melanopsin, the recording chamber was shrouded in black vinyl to block all extraneous light.
We had problems recording from ipRGCs from phosphonull-infected retinas. To increase our chances of recording from ipRGCs, we prepared retinas under dim red light, incubated the retina with 9-cis-retinal, minimized exposure to stray light, and minimized the duration of epifluorescent light exposure while identifying the cells. Unfortunately, these precautions did not lead to a noticeable improvement in the number of responsive cells in the C terminus phosphonull retinas, indicating that even short exposure to epifluorescent light causes saturation (and perhaps depolarization block), which renders ipRGCs unable to respond to subsequent light stimulation. Consistent with activation via epifluorescent light exposure, we observed that ipRGCs from WT-infected animals have a resting membrane potential of −54 mV (7.5 SEM), whereas ipRGCs from phosphonull-infected animals have a resting membrane potential of −46 mV (3.5 SEM).
MEA Recordings.
Mice were killed by cervical dislocation. Retinal preparation and MEA recording setups were as described (33, 34). WT and Opn4Cre/Cre + AAV2 (C terminus phosphonull) retinal pieces were used for analysis. Retinas from dark-adapted mice were dissected under infrared and red illumination and maintained for at least 20 min at 25 °C in Ringer buffer (124 mM NaCl, 5 mM KCl, 1.15 mM KH2PO4, 1.15 mM MgSO4, 2.5 mM CaCl2, 25 mM NaHCO3, 10 mM d-glucose) saturated with 95% O2 and 5% CO2. Synaptic blockade to eliminate rod/cone input to ipRGCs was achieved by perfusing the inhibitor mixture [250 μM DL-AP4, 50 μM picrotoxin, 10 μM DNQX, 10 μM strychnine, 100 μM carbenoxolone, 200 μM meclofenamic acid (MFA), a gap junction inhibitor, 50 µM tubocurarine] into the recording chamber for a duration of 10 min before beginning a recording.
We used a 60MEA100/10 multielectrode system, with an external ground electrode, adapted to a MEA amplifier and MCRack software (Multi Channel Systems; distributed by ALA Scientific). Blue-light stimulation with an intensity of ∼2.9 × 1015 photons per cm−2⋅s−1 from a Luxeon Rebel LXML-PB01-0040 blue LED (Philips Lumileds, Inc.) was driven by an Arduino micro controller (Adafruit, Inc.) and focused through the camera port of the microscope onto a square grid of 60 electrodes arranged in an 8 × 8 grid with the corner positions empty. The electrodes were titanium oxide with a 10-μm diameter and spaced at 100-μm intervals. Temperature control was achieved through an inline heating element and a head-stage plate heater, incorporated in the amplifier stage. The visual stimulation protocol consisted of a 30-s baseline (in darkness) acquisition, followed by a 5-s blue light stimulus and a 4-min and 30-s dark period, and it was synchronized with the MCRack recording software, using a transistor--transistor logic (TTL) synchronization signal pulse delivered by the Arduino device. Full waveform data were acquired at 25,000 Hz for all 60 channels. Field potential and noise were filtered offline using a filter based on the Eigendecomposition technique; spikes were detected using an adaptive threshold detector and sorted using a clustering algorithm.* WT and Pnull ipRGCs were diagnosed based on the persistent light response under synaptic pharmacological block by comparing their average firing frequency during the 30 s preceding and 60 s immediately after the light stimulus, using a k-means algorithm.
Cloning and Viral Constructs.
A floxed melanopsin WT gene and the CPΦ constructs, both with a C-terminal FLAG tag and N-terminal mRuby-P2A tag, were cloned into pAAV (Fig. S1B). The mRuby is a red fluorescent protein that acts as a quantitative visual marker of cells expressing melanopsin (38). The P2A is a viral peptide that will allow the expression of melanopsin fusion construct into a bicistronic transcript and will inhibit glycine–proline peptide bond formation during translation, resulting in two peptides (mRuby and melanopsin-FLAG) of equimolar proportions (39). The lack of peptide bond formation results in the addition of a proline residue to the amino terminus of melanopsin, but does not appear to affect trafficking deleteriously (as determined by staining of nonpermeabilized transfected cells with an antibody against an extracellular epitope) or function (based on in vitro calcium imaging studies) (Figs. S2 and S3). The viral vectors were tested for functionality (Fig. S2) and then packaged into AAV2 capsids. AAV2 has been extensively examined and exhibits tropism for neuronal cells in addition to a few other cell types, and it is particularly effective at infecting retinal ganglion cells following intravitreal injection (40–42).
In Vitro Calcium Imaging.
The in vitro calcium imaging protocol was conducted as described by Blasic et al. (14, 15). Viral vectors were transiently transfected into HEK293 and HEK293-Cre cells, and mammalian expression vectors were transiently transfected into HEK293 (Fig. S2). Four hours posttransfection, cells were split into a 96-well dish at a density of ∼80,000 cells per well and dark-adapted. Forty-eight hours after transfection, cells were treated with Fluo-4 AM (calcium-sensitive fluorescent dye) and incubated for 60 min. Following incubation, fluorescent measurements were made at excitation and emission wavelengths of 485 nm and 520 nm, respectively, using a fluorescent plate reader (Fig. S2).
Supplementary Material
Acknowledgments
We thank Joe Blasic, William Keenan, Evan Cameron, and all others members of the P.R.R. and S.H. laboratories for their helpful comments; Beverly Wu for the viral vector backbone; and Hiryanna Suja for technical assistance with vector packaging. This study was supported by NIH Grants R01EY019053 (to P.R.R.), GM076430 (to S.H.), and EY024452 (to S.H.); a grant from the Chapman–Perelman NeuroPsychoAnalytic Society (to R.L.B.); and a grant from the National Eye Institute Intramural Research Program (to T.C.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Ghahari A, Badea TC, 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, August 16–20, 2016, Orlando, FL.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611893114/-/DCSupplemental.
References
- 1.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95(1):340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Güler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter ML, et al. Shedding new light on opsin evolution. Proc Biol Sci. 2012;279(1726):3–14. doi: 10.1098/rspb.2011.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301(5632):525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 6.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 7.Ecker JL, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: Cellular diversity and role in pattern vision. Neuron. 2010;67(1):49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol. 2008;100(1):371–384. doi: 10.1152/jn.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29(2):476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582(Pt 1):279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Brown RL. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur J Neurosci. 2006;24(4):1117–1123. doi: 10.1111/j.1460-9568.2006.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 14.Blasic JR, Jr, Brown RL, Robinson PR. Light-dependent phosphorylation of the carboxy tail of mouse melanopsin. Cell Mol Life Sci. 2012;69(9):1551–1562. doi: 10.1007/s00018-011-0891-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blasic JR, Jr, et al. Identification of critical phosphorylation sites on the carboxy tail of melanopsin. Biochemistry. 2014;53(16):2644–2649. doi: 10.1021/bi401724r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mure LSS, et al. Melanopsin-encoded response properties of intrinsically photosensitive retinal ganglion cells. Neuron. 2016;90(5):1016–1027. doi: 10.1016/j.neuron.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci USA. 2008;105(41):16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas RJ, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299(5604):245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 19.Do MT, et al. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457(7227):281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4(6):621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 21.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20(6):989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 22.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36(9):457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendez A, et al. Rapid and reproducible deactivation of rhodopsin requires multiple phosphorylation sites. Neuron. 2000;28(1):153–164. doi: 10.1016/s0896-6273(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 24.Do MT, Yau KW. Adaptation to steady light by intrinsically photosensitive retinal ganglion cells. Proc Natl Acad Sci USA. 2013;110(18):7470–7475. doi: 10.1073/pnas.1304039110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sexton TJ, Van Gelder RN. G-protein coupled receptor kinase 2 minimally regulates melanopsin activity in intrinsically photosensitive retinal ganglion cells. PLoS One. 2015;10(6):e0128690. doi: 10.1371/journal.pone.0128690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emanuel AJ, Do MT. Melanopsin tristability for sustained and broadband phototransduction. Neuron. 2015;85(5):1043–1055. doi: 10.1016/j.neuron.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42(44):12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- 28.Walker MT, Brown RL, Cronin TW, Robinson PR. Photochemistry of retinal chromophore in mouse melanopsin. Proc Natl Acad Sci USA. 2008;105(26):8861–8865. doi: 10.1073/pnas.0711397105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chew KS, Schmidt TM, Rupp AC, Kofuji P, Trimarchi JM. Loss of gq/11 genes does not abolish melanopsin phototransduction. PLoS One. 2014;9(5):e98356. doi: 10.1371/journal.pone.0098356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48(6):1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Chen CK, et al. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc Natl Acad Sci USA. 1999;96(7):3718–3722. doi: 10.1073/pnas.96.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cahill H, Nathans J. The optokinetic reflex as a tool for quantitative analyses of nervous system function in mice: Application to genetic and drug-induced variation. PLoS One. 2008;3(4):e2055. doi: 10.1371/journal.pone.0002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuoka RL, et al. Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron. 2011;71(3):460–473. doi: 10.1016/j.neuron.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye X, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139(2):285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17(9):1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298(5601):2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 37.Ruby NF, et al. Role of melanopsin in circadian responses to light. Science. 2002;298(5601):2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 38.Kredel S, et al. mRuby, a bright monomeric red fluorescent protein for labeling of subcellular structures. PLoS One. 2009;4(2):e4391. doi: 10.1371/journal.pone.0004391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JH, et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One. 2011;6(4):e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye X, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Neuron. 2003;4(5):949–956. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grant CA, Ponnazhagan S, Wang XS, Srivastava A, Li T. Evaluation of recombinant adeno-associated virus as a gene transfer vector for the retina. Curr Eye Res. 1997;16(9):949–956. doi: 10.1076/ceyr.16.9.949.5046. [DOI] [PubMed] [Google Scholar]
- 42.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23(18):7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.