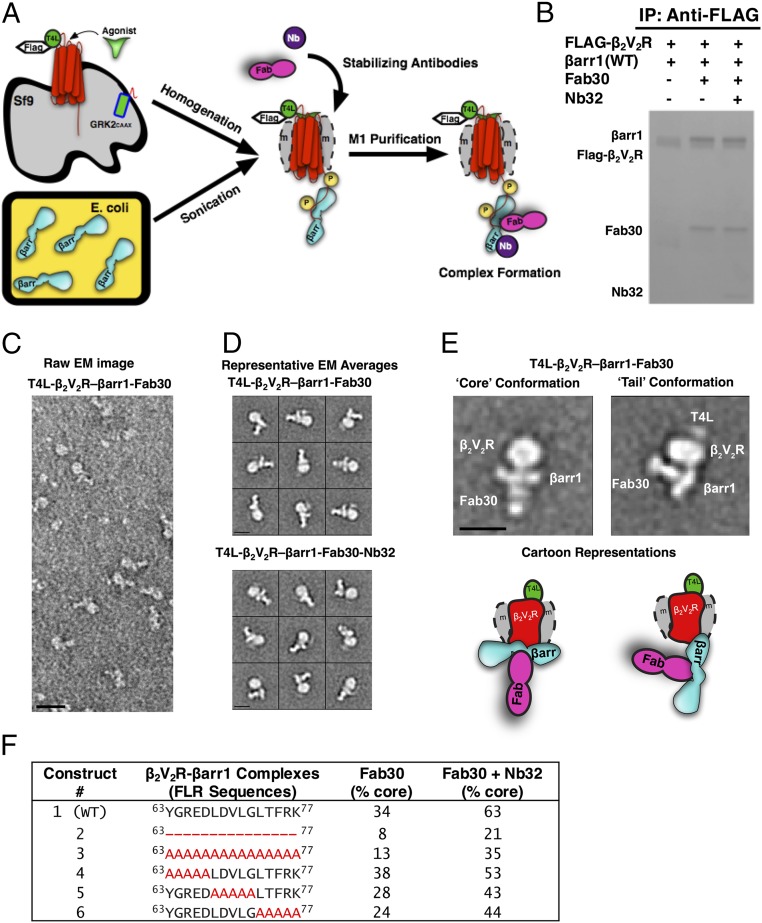
Fig. 1.
T4 lysozyme (T4L)β2V2R–βarr1 complexes formed and analyzed via EM with a newly developed functional purification method. (A) Schematic representation of the purification method to generate (T4L)β2V2R–βarr1 complexes. (B) Coomassie gel showing WT βarr1 interaction with (T4L)β2V2R in the absence or presence of conformation-stabilizing antibodies (Fab30, Nb32). IP, immunoprecipitation. (C) Representative negative-stain raw EM image of (T4L)β2V2R–βarr1–Fab30 complexes. (D) Class averages of the (T4L)β2V2R–βarr1–Fab30 complexes (Top) and (T4L)β2V2R–βarr1–Fab30–Nb32 complexes (Bottom) from negative-stain EM classification analysis. (E) Representative class averages (with cartoon representations) of the (T4L)β2V2R–βarr1–Fab30 complex in the tail and core conformations. (Scale bars: C, 20 nm; D and E, 10 nm.) (F) Summarized results of the different βarr1 FLR constructs tested for their ability to form the (T4L)β2V2R–βarr1–Fab30 core conformation in the presence or absence of Nb32. Note that the tail conformation encompasses all those (T4L)β2V2R–βarr1 complexes that are not in the core conformation.