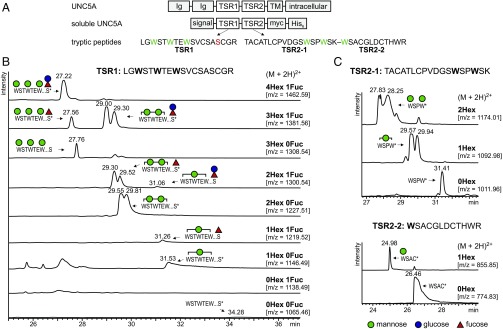
Fig. 1.
C-mannosylation pattern of UNC5A in wild-type CHO cells. (A) Soluble UNC5A construct and proteolytic fragments analyzed by mass spectrometry. Membrane-bound UNC5A contains two Ig domains, and two thrombospondin type 1 repeats (TSRs) in the extracellular part of the protein. The two TSRs were used in an expression construct that allowed secretion of the protein fragment. Analyzed trypsin proteolytic fragments with putative C-mannosylation (green) and O-fucosylation (red) sites are depicted. TM, transmembrane domain. (B) Extracted ion chromatograms of observed glycoforms of the UNC5A TSR1 peptide. For signals marked by *, MS/MS spectra confirmed the nature of the peptide and presence of C-mannose (SI Appendix, Fig. S1). Based on MS/MS data and elution time, peaks were attributed to the indicated glycoforms. All graphs are shown with equal intensities (12,000 counts per second). (C) Extracted ion chromatograms of observed glycoform masses of the UNC5A TSR2 peptides. Corresponding MS/MS spectra are shown in SI Appendix, Fig. S2 A and B for the first and second peptides of TSR2, respectively.