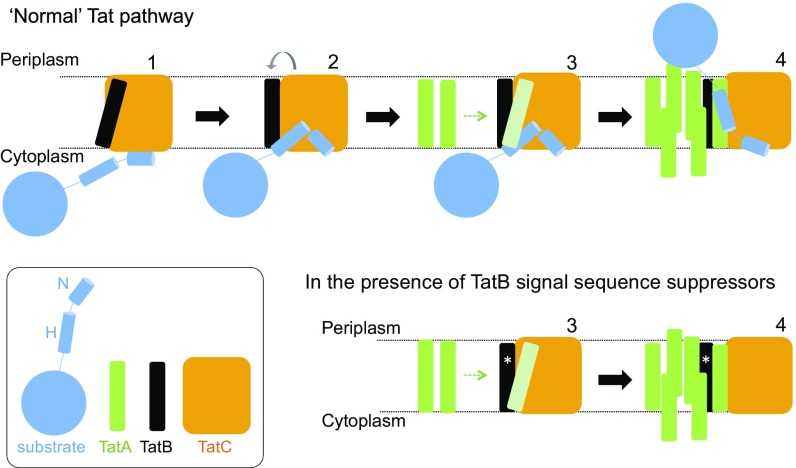
Fig. 8.
Tat translocases containing TatB suppressor variants may more readily transition to the signal peptide-activated state. (Upper) Model for Tat transport. A signal peptide bound through its n-region to the cytoplasmic surface of TatC (step 1) transitions to a deep binding mode (step 2). The deep insertion of the signal peptide displaces TatB from its resting state binding site on TatC (gray arrow). TatB movement allows polymerization of TatA to be nucleated (step 3). The substrate passes across the membrane facilitated by the TatA oligomer (step 4). (Lower) TatB variants that suppress signal sequence defects (represented with an asterisk, *) may be more easily displaced from the resting-state binding site. The TatB variants appear to be on a continuum with TatB F13Y pushing the Tat system into an assembled state (step 4), whereas Tat systems harboring the weaker suppressing variants are more likely to correspond to step 3.