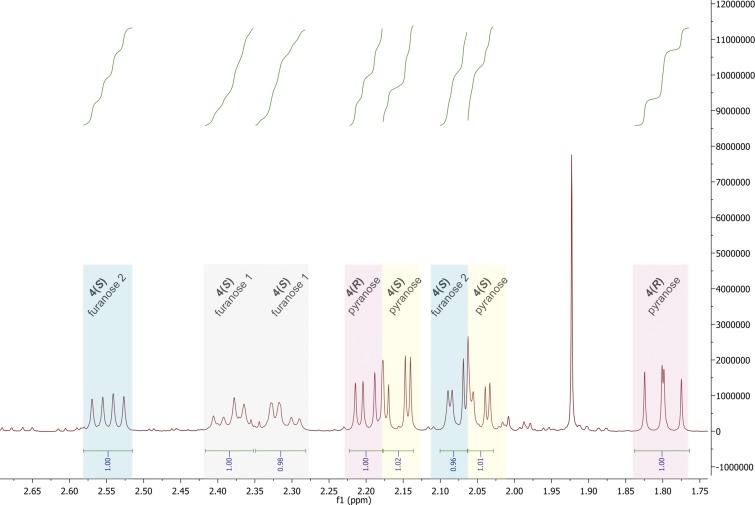
Fig. S3.
Region 1.75–2.65 ppm of the proton NMR spectrum of DHA. A sample of DHA was purified by anion exchange. Fractions containing DHA were pooled and freeze-dried. DHA was dissolved in D2O for analysis by 500 MHz 1H NMR spectroscopy. Two diastereomeric products were observed in the NMR spectrum: (4R, -5S, -6R) and (4S, -5S, -6R) DHA. The peaks in the region 1.75–2.65 ppm were used to assign these forms because these peaks correspond to the diastereotopic pair of protons formed during the aldol condensation at C3–C4. The (4R) diastereomer was seen to adopt one pyranose ring form (peaks corresponding to this form are highlighted in pink), and the (4S) diastereomer was seen to adopt three different ring forms: one pyranose (highlighted in yellow) and two distinct furanose (highlighted in gray and blue) forms.