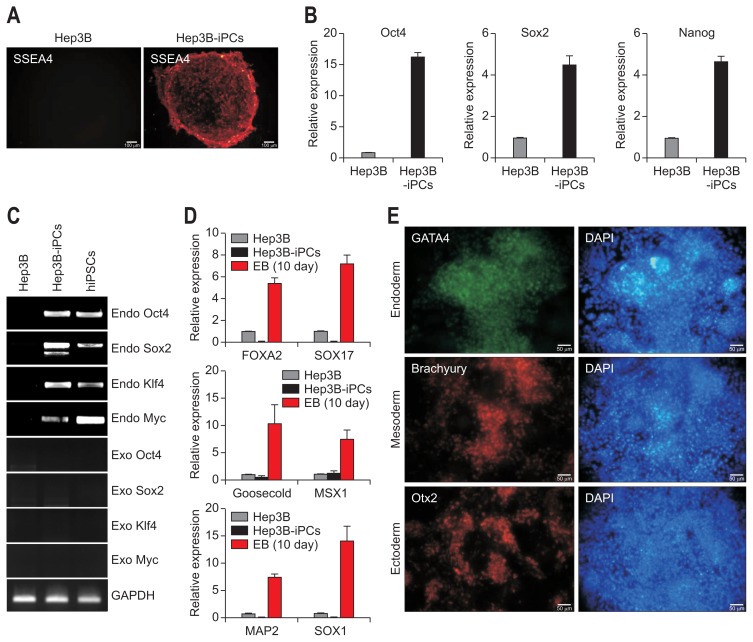
Fig. 3.
Analysis of gene expression related to the pluripotency and in vitro tri-lineage differentiation potential of Hep3B-iPC cells. Analysis of pluripotent marker expression in Hep3B and Hep3B-iPC cells at passage 4. (A) Live staining with antibody against stage-specific embryonic antigen-4 (SSEA4), which is a pluripotent marker. Scale bar=100 μm. (B) Quantitative real-time polymerase chain reaction (PCR) analysis with primers specific to pluripotent markers, including Oct4, Sox2, and Nanog. The mRNA expression values were normalized to GAPDH and are shown as the mean±SD of triplicate experiments, expressed relative to Hep3B. Evidence of the differentiation potential of Hep3B-iPCs into tri-lineage-type cells in vitro. (C) Reverse transcription polymerase chain reaction for exogenous and endogenous gene expression of the four reprogramming factors. (D) Quantitative real-time PCR analysis with primers specific to endoderm (Foxa2 and Sox17), mesoderm (Goosecoid and Msx1), and ectoderm (Map2 and Sox1). The embryonic body (EB) was formed from Hep3B-iPCs and cultured for 10 days. The mRNA expression values were normalized to glyceraldehydes 3-phosphate dehydrogenase (GAPDH) and are shown as the mean±SD of triplicate experiments, expressed relative to Hep3B. (E) Immunostaining with antibodies against the three germ layers, namely, antibodies to GATA4 (green, endoderm), Brachyury (red, mesoderm), and Otx2 (red, ectoderm). Nuclei were double-stained with DAPI (blue). Scale bar=50 μm. Tri-lineage differentiation of Hep3B-iPCs was performed as indicated in the materials and methods.
iPC, induced pluripotent cancer; iPSCs, induced pluripotent stem cell from fibroblasts.