FIGURE 1.
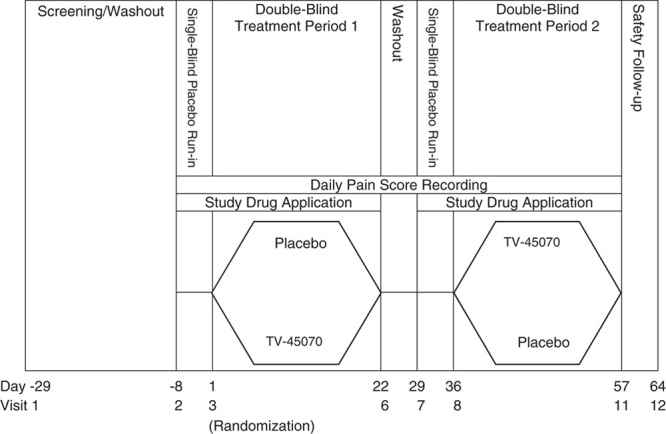
Study design. Screening/washout lasted up to 3 weeks (day-29 to day-8). The first single-blind, placebo run-in period was 1 week in duration (day-7 to day-1). At randomization (visit 3), patients were randomized in a crossover design to 1 of 2 treatment sequences: TV-45070/placebo or placebo/TV-45070. The duration of TP1 was 3 weeks. During the 1-week between-treatment washout period (between visits 6 and 7), study medication was not applied, but patients continued to record pain scores using the interactive voice response system. The second single-blind, placebo run-in period was 1 week in duration (day-29 to day-35). In TP2, patients received the medication that was not received during TP1. The duration of TP2 was 3 weeks. One week after the end of TP2, patients returned for a follow-up visit (visit 12). TP1 indicates treatment period 1; TP2, treatment period 2.
