Abstract
Background
Acyclovir is used to treat herpes simplex virus (HSV) disease in infants. Treatment with high dose acyclovir, 60 mg/kg/day, is recommended; however, the safety of this dosage has not been assessed in the past 15 years, and this dosage is not currently approved for infants by the US Food and Drug Administration.
Methods
We included infants with neonatal HSV disease treated with ≥14 days of intravenous acyclovir starting in the first 120 days of life admitted to 1 of 42 neonatal intensive care units managed by the Pediatrix Medical Group from 2002–2012. We determined the frequency and proportion of infants with clinical and laboratory adverse events (AEs) as well as the number and proportion of infant days with laboratory AEs occurring during acyclovir exposure.
Results
We identified 89 infants during the study period with 1658 days of acyclovir exposure. Almost all received high-dose acyclovir therapy (79/89, 89%). The most common clinical AEs were hypotension and seizure, both occurring in 9% of infants. Thrombocytopenia was the most common laboratory AE occurring in 25% of infants and on 9% of infant-days. Elevated creatinine occurred in 2% of infants and 0.2% of infant-days and no infants developed renal failure requiring dialysis. Overall, 45% of infants had ≥1 AE.
Conclusions
In this cohort of infants treated during the high-dose acyclovir era, AEs were common, but usually not severe. Many of the AEs reported in this cohort may be related to the underlying infection rather than due to acyclovir exposure.
Keywords: herpes simplex virus, adverse events, neutropenia, renal failure
INTRODUCTION
Neonatal herpes simplex virus (HSV) disease is an uncommon, but serious infection associated with significant morbidity and mortality.1 Neonatal HSV disease has three distinct presentations: disseminated disease, localized infection of the skin, eyes or mucous membranes (SEM), and infection of the central nervous system (CNS).2,3 Prognosis and treatment duration vary depending on the presentation.
Acyclovir has been considered the treatment of choice for neonatal HSV disease since 1991.4 Based on a prospective study including 107 infants treated with acyclovir, the dose approved by the United States Food and Drug Administration (FDA) is 10 mg/kg/dose administered every 8 hours.4,5 However, in a more recent, prospective, non-randomized trial, the 88 infants who received higher dosing (20 mg/kg/dose administered every 8 hours) had lower mortality than the 107 infants who received 10 mg/kg.1 The American Academy of Pediatrics recommends that high-dose (HD) acyclovir (20 mg/kg/dose) be used for the treatment of neonatal HSV disease.6
Acyclovir has been associated with nephrotoxicity, neutropenia and neurotoxicity in prior studies of children and adults.7–9 The frequency of these events in infected infants is unclear. We sought to evaluate the safety of acyclovir in hospitalized infants with neonatal HSV disease during the era of HD acyclovir use.
MATERIALS AND METHODS
Study Population
We identified all infants with neonatal HSV disease discharged from 42 neonatal intensive care units (NICUs) managed by the Pediatrix Medical Group from 2002 to 2012. The Pediatrix Medical Group maintains an electronic medical record that prospectively captures information from notes generated by clinicians on all infants cared for by the group. Data on multiple aspects of care are entered into the system to generate admission notes, daily progress notes, procedure notes, and discharge summaries. Information is collected regarding maternal history and demographics, medications, respiratory support, laboratory results, culture results, procedures, daily growth parameters and diagnoses.
We included infants with HSV disease who were ≤120 days postnatal age when acyclovir was started and received ≥14 days of acyclovir or died while on acyclovir therapy. Fourteen days of acyclovir exposure was used in an attempt to limit our study population to infants with HSV disease; since 14 days is the shortest duration of therapy recommended for HSV6, shorter courses likely reflected empirical therapy while awaiting diagnostic studies and not actual infection. HSV disease was considered to be present when either a clinical diagnosis of HSV disease was assigned by the treating physician or when at least one of the following was positive: HSV DNA polymerase chain reaction (PCR) or culture from blood, cerebrospinal fluid (CSF), skin, eyes, mouth or rectum. Infants were considered to have disseminated disease if there was a positive virologic test from the blood, an aspartate aminotransferase level ≥100 units/L or a diagnosis of disseminated intravascular coagulation (DIC) within 3 days before to 3 days after the start of acyclovir therapy. Infants were considered to have SEM disease if there was a positive virologic test from the skin, eyes, mouth or rectum but not the CSF and the criteria for disseminated disease were not met andCNS disease if there was a positive virologic test from the CSF and the criteria for disseminated disease were not met. We excluded infants for whom the dose of acyclovir was unknown. If an infant received multiple courses of acyclovir treatment, only the first course was included in the analysis.
Definitions
We considered a clinical adverse event (AE) to be present if a diagnosis of interest was made on a day with acyclovir exposure and was not present in the 3 days prior to starting acyclovir. Laboratory AEs were included if the event occurred on a day with acyclovir exposure. All AEs were defined a priori.10 Clinical AEs included: hypotension; seizures; and renal failure requiring dialysis. Hypotension was defined as exposure to dopamine, dobutamine, epinephrine, norepinephrine or milrinone; actual blood pressures were not available. Seizures were considered to be present when diagnosed by a clinician; electroencephalogram confirmation was not required. Laboratory AEs were categorized as AEs or severe AEs (Table 2). Small for gestational age status was defined as previously described.11
Table 2.
Laboratory adverse and severe adverse events
| Adverse Event | Severe Adverse Event | |||||
|---|---|---|---|---|---|---|
| Serum electrolytes | Infants N=89 (%) | Infant-Days N=1658 (%) | Infants N=89 (%) | Infant-Days N=1658 (%) | ||
| Hypernatremia | > 150 mmol/L | 6 (7) | 7 (<1) | > 160 mmol/L | 0 (0) | 0 (0) |
| Hyponatremia | < 125 mmol/L | 4 (4) | 6 (<1) | < 115 mmol/L | 0 (0) | 0 (0) |
| Hyperkalemia | > 6.5 mmol/L | 15 (17) | 23 (1) | > 8.0 mmol/L | 4 (4) | 5 (<1) |
| Hypokalemia | < 3 mmol/L | 5 (6) | 7 (<1) | < 2.0 mmol/L | 0 (0) | 0 (0) |
| Renal dysfunction | ||||||
| Elevated BUN | > 60 mg/dL | 0 (0) | 0 (0) | > 100 mg/dL | 0 (0) | 0 (0) |
| Elevated creatinine | > 1.7 mg/dL | 2 (2) | 4 (<1) | > 3.0 mg/dL | 0 (0) | 0 (0) |
| Complete blood count | ||||||
| Neutropenia | < 500/mm3 | 5 (6) | 5 (<1) | < 100/mm3 | 0 (0) | 0 (0) |
| Leukopenia | < 5000/mm3 | 14 (16) | 36 (2) | < 2000/mm3 | 1 (1) | 2 (<1) |
| Thrombocytopenia | < 100,000/mm3 | 22 (25) | 147 (9) | < 20,000/mm3 | 3 (3) | 4 (<1) |
| Any laboratory adverse event | 37 (42) | 193 (12) | 8 (9) | 11 (1) | ||
Statistical Analysis
We described the demographic characteristics of infants who received acyclovir using counts and proportions for categorical variables and medians with 25th and 75th percentiles for continuous variables. The number and proportion of infants with each clinical AE were determined. The number and proportion of infants and infant-days with each laboratory AE and serious adverse event (SAE) were also determined. This same analysis was repeated for infants <1500 g birth weight. We compared the proportion of infants <1500 g birth weight who experienced each AE and SAE to that of infants ≥1500 g birth weight using a chi-square test. We compared the proportion of infants who were diagnosed with each type of HSV disease who had each clinical and laboratory AE. We determined the number and proportion of infants receiving standard dose (<35 mg/kg/day), intermediate dose (35–50 mg/kg/day) and HD (≥50 mg/kg/day) acyclovir. We used STATA 14.0 (College Station, TX) to conduct all analyses. This study was approved by the Duke Institutional Review Board with a waiver of informed consent.
RESULTS
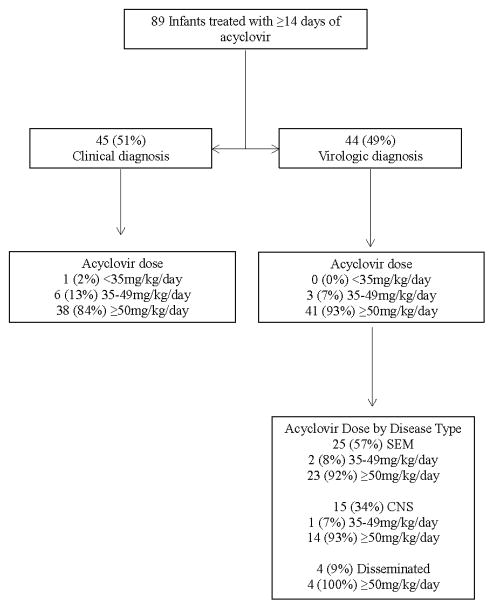
We identified 89 infants with neonatal HSV disease treated with acyclovir for a total of 1658 days (Table 1). The median gestational age was 34 weeks (25th, 75th percentile: 31, 38) and the median birth weight was 2000 g (1485, 3065). HSV disease was diagnosed clinically in 45 (51%) infants (Figure 1). Of the 44 (49%) infants with a virologic diagnosis, 22 (50%) had SEM disease, 11 (25%) had CNS disease and 11 (25%) had disseminated disease. An additional infant with a clinical diagnosis was diagnosed with disseminated disease on the basis of a diagnosis of DIC at presentation. The median postnatal age on the first day of acyclovir exposure was 5 days (2, 8) and the median duration of acyclovir therapy was 18 days (15, 22). The median daily dose of acyclovir was 60mg/kg (60, 66) with almost all infants receiving HD acyclovir therapy (79/89, 89%). Only 1 infant received <35 mg/kg/day. The remaining 9 infants (10%) received intermediate dose acyclovir.
Table 1.
Demographics
| N=89 (%) | |
|---|---|
| Gestational age, weeks | |
| ≤25 | 5 (6) |
| 26–28 | 8 (9) |
| 29–32 | 25 (28) |
| 33–36 | 14 (16) |
| ≥37 | 37 (42) |
| Birth weight, g | |
| <1000 | 9 (10) |
| 1000–1499 | 14 (16) |
| 1500–2499 | 28 (31) |
| 2500–3499 | 28 (31) |
| ≥3500 | 10 (11) |
| Apgar score at 5 minutes | |
| 0–3 | 1 (1) |
| 4–6 | 11 (13) |
| 7–10 | 74 (86) |
| Race/ethnicity | |
| White | 45 (52) |
| African-American | 26 (30) |
| Hispanic | 14 (16) |
| Other | 1 (1) |
| Male | 43 (48) |
| Inborn | 69 (78) |
| Cesarean section | 40 (45) |
| Small for gestational age | 8 (9) |
| Source of HSV diagnosis | |
| Clinical diagnosis | 45 (51) |
| Virologic diagnosis | 44 (49) |
| Age at 1st acyclovir dose, days | |
| <7 | 58 (66) |
| 7–13 | 19 (22) |
| 14–28 | 10 (11) |
| >28 | 1 (1) |
| Duration of therapy, days | 18 (15, 22)* |
median (25th, 75th percentile)
Figure 1.
Neonatal herpes simplex virus disease treated with ≥14 days of a cyclovir.
At least one AE was diagnosed in nearly half of all infants exposed to acyclovir (40/89, 45%). Laboratory AEs occurred in 37/89 (42%) of infants and 193/1658 (12%) of infant-days. Thrombocytopenia was the most common laboratory AE (Table 2). Hematologic AEs were common with 25% (22/89) of infants experiencing thrombocytopenia and 16% (14/89) of infants developing leukopenia. Thrombocytopenia and leukopenia occurred after a median of 2 days (range: 1–9) and 1 day (range: 1–8) of acyclovir exposure, respectively. Neutropenia occurred in 6% (5/89) of infants. Most of these infants (3/5, 60%) received granulocyte colony stimulating factor for treatment of their neutropenia. Mild elevation of creatinine levels occurred in 2% (2/89) of infants. Serious laboratory AEs were uncommon. Clinical AEs were less common (15/89, 17%). The most common clinical AEs were hypotension (8/89, 9%) and seizure (8/89, 9%) (Table 3). Five of the 8 infants who had a seizure had virologic confirmation of HSV; 2 had CNS disease, 2 had disseminated disease and 1 had SEM disease. Other clinical AEs were rare. There were no cases of nephrotoxicity requiring dialysis. Three (4%) infants died; 2 of these had a virologic diagnosis;1 had CNS disease and 1 had disseminated disease. The frequencies of most AEs were similar for infants with and without virologic confirmation of HSV disease; leukopenia and thrombocytopenia were more frequent in infants with a virologic diagnosis (Table 4).
Table 3.
Clinical adverse events
| Adverse Event | Infants N=89 (%) | Duration prior to AE, median (min, max) |
|---|---|---|
| Hypotension requiring inotropes | 8 (9) | 2 (1, 13) |
| Seizure | 8 (9) | 1 (1, 39) |
| Renal failure requiring dialysis | 0 (0) | - |
| Any clinical adverse event | 15 (17) | - |
Table 4.
Clinical and laboratory adverse events for infants a laboratory diagnosis of herpes simplex virus disease
| Disseminated Disease N=12 | SEM Disease N=22 | CNS Disease N=11 | P | |
|---|---|---|---|---|
| Clinical Adverse Event | ||||
| Hypotension requiring inotropes | 2 (17%) | 4 (18%) | 0 (0%) | 0.45 |
| Seizure | 2 (17%) | 1 (5%) | 2 (18%) | 0.28 |
| Renal failure requiring dialysis | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Any clinical adverse event | 3 (25%) | 5 (23%) | 2 (18%) | >0.99 |
| Laboratory Adverse Event | ||||
| Hypernatremia >150 mmol/L | 3 (25%) | 1 (5%) | 1 (9%) | 0.22 |
| Hyponatremia <125 mmol/L | 1 98%) | 2 (9%) | 0 (0%) | 0.80 |
| Hyperkalemia >6.5 mmol/L | 0 (0%) | 3 (14%) | 4 (36%) | 0.05 |
| Hypokalemia <3 mmol/L | 2 (17%) | 2 (9%) | 0 (0%) | 0.45 |
| Elevated BUN >60 mg/dL | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Elevated creatinine > 1.7 mg/dL | 0 (0%) | 1 (5%) | 1 (9%) | 0.73 |
| Neutropenia < 500/mm3 | 2 (17%) | 0 (0%) | 2 (18%) | 0.08 |
| Leukopenia < 5000/mm3 | 6 (50%) | 4 (18%) | 1 (9%) | 0.08 |
| Thrombocytopenia < 100,000/mm3 | 9 (75%) | 5 (23%) | 3 (27%) | 0.01 |
| Any laboratory adverse event | 9 (75%) | 8 (36%) | 7 (64%) | 0.08 |
| Any adverse event | 9 (75%) | 9 (41%) | 7 (64%) | 0.13 |
We found that AEs occurred more frequently in infants with lower birth weights. Seventy percent (16/23) of infants <1500 g had at least 1 AE; 36% (24/66) of infants ≥1500 g had at least 1 AE, P=0.006. The most common AE for infants <1500 g was thrombocytopenia, occurring in 5/23 (22%) infants and 86/450 (19%) infant-days.
DISCUSSION
This study provides safety information for a large cohort of infants treated with acyclovir for neonatal HSV disease. We found that clinical and laboratory events were common but usually not severe. Most of the infants received HD therapy. Only 1 infant (1%) received the dose recommended in the FDA label (30 mg/kg/day).
Nephrotoxicity is the AE related to acyclovir exposure that is most concerning.12 Damage to the renal tubules is thought to occur when acyclovir crystals precipitate leading to an obstructive nephropathy.12 A retrospective case-control study of 373 infants and children treated with intravenous acyclovir found that nephrotoxicity defined as ≥25% decrease in glomerular filtration rate occurred in 35% of patients.13 They also noted that the risk of nephrotoxicity was increased with doses of acyclovir >15 mg/kg, age >8 years, and concomitant ceftriaxone use.13 Another study that included children age 1–15 years found that 70% of those treated simultaneously with acyclovir and ceftriaxone developed nephrotoxicity defined as serum creatinine >105% of the baseline creatinine measurement.14 However, nephrotoxicity defined as a change in the post-acyclovir glomerular filtration rate compared to baseline was not observed in 14 neonates treated with a mean of 45 mg/kg/day of acyclovir 7; furthermore, ceftriaxone is rarely used in young infants. Avoiding fluid restriction in infants with HSV, a common practice in the past, has likely contributed to the reduction in acyclovir-associated nephrotoxicity in infants.7 An elevated creatinine occurred in only 2% of infants in our cohort and none of our patients required dialysis. This is less frequent than was previously described in the prospective study evaluating HD acyclovir in infants (6%).1 Electrolyte abnormalities occurred in as many as 17% of infants in our study. It is possible that these abnormalities were related to the underlying HSV disease or caused by abnormal renal function in the absence of creatinine changes.
Case reports have suggested that neutropenia can occur with prolonged or repeated acyclovir courses.15,16 A prospective study found that an absolute neutrophil count (ANC) <1000/mm3 occurred in 21% of infants treated with HD acyclovir.1 An older study found that 0 of 107 infants treated with SD acyclovir had a white blood cell count <2500/mL.4 Similarly, neutropenia was not seen in any of 29 children ages 1 month to 18 years treated with HD acyclovir.17 Six percent of infants in our study had neutropenia (ANC<500/mm3) and 11% (10/89) had an ANC<1000/mm3 providing further support that the incidence of neutropenia while exposed to acyclovir is low. In all of these studies, the infants recovered uneventfully with continuation of acyclovir therapy or after completion of the treatment course whether oral or intravenous acyclovir was administered.1
Acyclovir-associated neurotoxicity has been reported in adults, particularly the elderly and those with severe renal impairment.18,19 Prior reports of acyclovir-associated neurotoxicity indicate that prominent signs are delusions, hallucinations and involuntary movements.8,18 Seizures have not specifically been described and may be more indicative of CNS HSV disease than acyclovir-associated neurotoxicity.8 A retrospective study of infants and children found that 50% of the 6 neonates and 22% of the 32 non-neonates had seizures at the time of presentation.20 Another retrospective study of 26 infants with CNS HSV disease found that seizures occurred in 54% of patients; in 35%, the seizures were present at presentation.21 A clinical trial that enrolled 186 infants with neonatal HSV disease found that seizures were present in 57% of infants with CNS disease and in 22% of infants with disseminated disease; overall 27% of infants had seizures at presentation.22 This suggests that a significant proportion of seizures are likely due to HSV disease and not necessarily due to acyclovir exposure. In our analysis, we attempted to differentiate seizures due to HSV disease from those occurred as an AE related to acyclovir exposure by only including seizures that began after acyclovir therapy had been initiated but it is likely that some seizures beginning after the start of acyclovir therapy were still due to CNS HSV disease. In our cohort, 9% of infants had seizures with onset after acyclovir was started and at least half of these had CNS disease.
It is likely that the FDA-approved acyclovir dose has not been updated to reflect the improved efficacy with HD therapy because of a perceived lack of safety data. However, in addition to our study, two prior studies evaluating the overall safety of HD acyclovir have been previously conducted. A prospective study comparing 66 infants treated with HD acyclovir to 13 infants treated with intermediate dose acyclovir (45 mg/kg/day) found that laboratory abnormalities were not statistically different between the two groups.1 The other study was a retrospective comparison of SD versus HD acyclovir for the treatment of encephalitis. Children aged 3 months to 12 years were included; there were no infants with neonatal HSV disease included. AEs were not different between the 32 children receiving SD acyclovir and the 29 children receiving HD acyclovir.17 A population pharmacokinetic study that included 22 premature infants found that, although 66% of infants experienced an AE, none were thought to be related to acyclovir exposure.23 Many of these infants were treated with doses even higher than 80mg/kg/day.23 We were not able to compare the incidence of AEs with SD versus HD because only 1 patient in our cohort received SD therapy.
Based on the results of this study and prior prospective studies, all infants with suspected or confirmed neonatal HSV disease should receive parenteral acyclovir at 20mg/kg/dose. The duration and optimal dosing of oral suppressive therapy following the completion of treatment is outside the scope of the current evaluation but remains an important area of ongoing research. Our study is limited by the retrospective nature of the analysis. Laboratory assessments were obtained at the discretion of the treating physician rather than systematically. Similarly, diagnostic evaluations for clinical AEs were conducted at the discretion of the treating physician. Half of the infants in our cohort did not have virologic data available, which is a major weakness of this report. This may be because these infants had testing performed at another hospital and were transferred to a contributing site, or because the data available in the database are solely from the physician notes and do not include laboratory data directly. Fewer infants than expected had disseminated HSV disease. This may have occurred because blood PCR testing is often performed by reference labs; the results may have been recorded in a different manner than tests performed at the treating hospital or the blood may not have been tested in some cases due to the increased effort required to send the test. It is possible that our findings represent some AEs that were due to HSV disease itself and were not caused by acyclovir exposure. More than half of the infants in our cohort were born preterm such that our results may not be generalizable to all infants. However, since we would not expect a medication to be less safe in term infants than in preterm infants, acyclovir is likely at least as safe as demonstrated in our cohort.
Acknowledgments
Sources of Funding
This work was funded under National Institute of Child Health and Human Development (NICHD) contract HHSN275201000003I for the Pediatric Trials Network, as well as NICHD grant 1R25HD076475. This study used Clinical and Translational Science Award biostatistical services through the Division of Pediatric Quantitative Sciences (NIH-5UL-1RR024128-01).
Footnotes
Conflicts of Interest
C.P.H. receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001117). D.K.B. receives support from the National Institutes of Health (NIH) (award 2K24HD058735-06, National Center for Advancing Translational Sciences award UL1TR001117, National Institute of Child Health and Human Development contract HHSN275201000003I, and National Institute of Allergy and Infectious Diseases contract HHSN272201500006I); he also receives research support from Cempra Pharmaceuticals (sub-award to HHSO100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. P.B.S. receives salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Child Health and Human Development (1R21HD080606-01A1, HHSN275201000003I and 1R01-HD081044-01), and the Food and Drug Administration (1R18-FD005292-01); he also receives research support from Cempra Pharmaceuticals (sub-award to HHS0100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). J.E.E receives support from the National Institute of Child Health and Human Development of the National Institutes of Health under award number 5T32HD060558. For the remaining authors, none were declared.
References
- 1.Kimberlin DW, Lin CY, Jacobs RF, et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001 Aug;108(2):230–238. doi: 10.1542/peds.108.2.230. [DOI] [PubMed] [Google Scholar]
- 2.Pinninti SG, Kimberlin DW. Neonatal herpes simplex virus infections. Pediatric clinics of North America. 2013 Apr;60(2):351–365. doi: 10.1016/j.pcl.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA: the journal of the American Medical Association. 2006 Aug 23;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 4.Whitley R, Arvin A, Prober C, et al. A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection. Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med. 1991 Feb 14;324(7):444–449. doi: 10.1056/NEJM199102143240703. [DOI] [PubMed] [Google Scholar]
- 5.APP Pharmaceuticals L. Acyclovir Sodium Injectiuon: For Intravenous Infusion Only. 2012. [Google Scholar]
- 6.Pediatrics AAo. Herpes simplex. In: DWK, MTB, MAJ, SSL, editors. Red Book: 2015 Report of the Committee on Infectious Diseases. 30. Elk Grove Village, IL: American Academy of Pediatrics; 2015. pp. 432–445. [Google Scholar]
- 7.Schreiber R, Wolpin J, Koren G. Determinants of aciclovir-induced nephrotoxicity in children. Paediatr Drugs. 2008;10(2):135–139. doi: 10.2165/00148581-200810020-00008. [DOI] [PubMed] [Google Scholar]
- 8.Gentry J, Peterson C. Death delusions and myoclonus: acyclovir toxicity. Am J Med. 2015 Mar 14; doi: 10.1016/j.amjmed.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Kimberlin DW, Whitley RJ, Wan W, et al. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N Engl J Med. 2011 Oct 6;365(14):1284–1292. doi: 10.1056/NEJMoa1003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.England A, Wade K, Smith PB, Berezny K, Laughon M Committee BPfCAPTNAC. Optimizing operational efficiencies in early phase trials: The Pediatric Trials Network experience. Contemp Clin Trials. 2016 Mar;47:376–382. doi: 10.1016/j.cct.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010 Feb;125(2):e214–224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 12.Sawyer MH, Webb DE, Balow JE, Straus SE. Acyclovir-induced renal failure. Clinical course and histology. Am J Med. 1988 Jun;84(6):1067–1071. doi: 10.1016/0002-9343(88)90313-0. [DOI] [PubMed] [Google Scholar]
- 13.Rao S, Abzug MJ, Carosone-Link P, et al. Intravenous Acyclovir and Renal Dysfunction in Children: A Matched Case Control Study. The Journal of pediatrics. 2015 Feb 21; doi: 10.1016/j.jpeds.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Vomiero G, Carpenter B, Robb I, Filler G. Combination of ceftriaxone and acyclovir - an underestimated nephrotoxic potential? Pediatr Nephrol. 2002 Aug;17(8):633–637. doi: 10.1007/s00467-002-0867-5. [DOI] [PubMed] [Google Scholar]
- 15.Feder HM, Jr, Goyal RK, Krause PJ. Acyclovir-induced neutropenia in an infant with herpes simplex encephalitis: case report. Clin Infect Dis. 1995 Jun;20(6):1557–1559. doi: 10.1093/clinids/20.6.1557. [DOI] [PubMed] [Google Scholar]
- 16.Tuncer AM, Evis B, Kunak B, Akcayoz N, Ertem U. Erythroblastopenia and leukopenia in the patient with severe herpes zoster treated with intravenous acyclovir. Turk J Pediatr. 1989 Oct-Dec;31(4):317–321. [PubMed] [Google Scholar]
- 17.Kendrick JG, Ensom MH, Steer A, White CT, Kwan E, Carr RR. Standard-dose versus high-dose acyclovir in children treated empirically for encephalitis: a retrospective cohort study of its use and safety. Paediatr Drugs. 2014 Jun;16(3):229–234. doi: 10.1007/s40272-014-0066-4. [DOI] [PubMed] [Google Scholar]
- 18.Asahi T, Tsutsui M, Wakasugi M, et al. Valacyclovir neurotoxicity: clinical experience and review of the literature. Eur J Neurol. 2009 Apr;16(4):457–460. doi: 10.1111/j.1468-1331.2008.02527.x. [DOI] [PubMed] [Google Scholar]
- 19.Hellden A, Odar-Cederlof I, Diener P, et al. High serum concentrations of the acyclovir main metabolite 9-carboxymethoxymethylguanine in renal failure patients with acyclovir-related neuropsychiatric side effects: an observational study. Nephrol Dial Transplant. 2003 Jun;18(6):1135–1141. doi: 10.1093/ndt/gfg119. [DOI] [PubMed] [Google Scholar]
- 20.Schleede L, Bueter W, Baumgartner-Sigl S, et al. Pediatric herpes simplex virus encephalitis: a retrospective multicenter experience. J Child Neurol. 2013 Mar;28(3):321–331. doi: 10.1177/0883073812471428. [DOI] [PubMed] [Google Scholar]
- 21.Kotzbauer D, Andresen D, Doelling N, Shore S. Clinical and laboratory characteristics of central nervous system herpes simplex virus infection in neonates and young infants. Pediatr Infect Dis J. 2014 Nov;33(11):1187–1189. doi: 10.1097/INF.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 22.Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001 Aug;108(2):223–229. doi: 10.1542/peds.108.2.223. [DOI] [PubMed] [Google Scholar]
- 23.Sampson MR, Bloom BT, Lenfestey RW, et al. Population pharmacokinetics of intravenous acyclovir in preterm and term infants. Pediatr Infect Dis J. 2014 Jan;33(1):42–49. doi: 10.1097/01.inf.0000435509.75114.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones CA, Raynes-Greenow C, Isaacs D. Population-based surveillance of neonatal herpes simplex virus infection in Australia, 1997–2011. Clin Infect Dis. 2014 Aug 15;59(4):525–531. doi: 10.1093/cid/ciu381. [DOI] [PubMed] [Google Scholar]