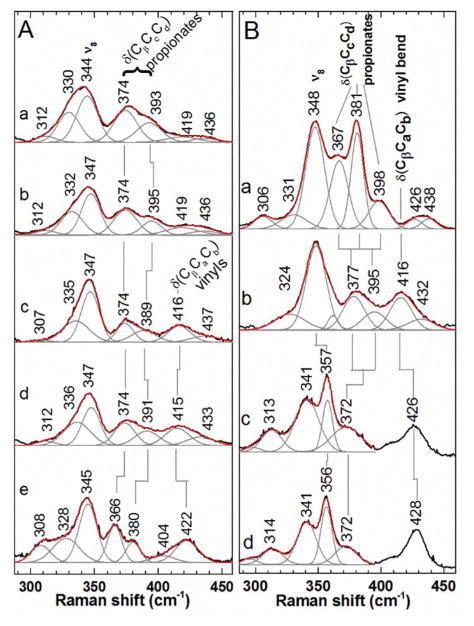
Figure 3. Low frequency rR spectra report on the core and peripheral environment of hemes bound to decarboxylases.
A) The 406.7-nm excited rR spectra of ferric forms of a) coproheme: Sa decarboxylase, b) coproheme:Gs decarboxylase c) heme b:Sa decarboxylase, d) heme b:Gs decarboxylase, and e) DaCld(R183Q). Ferric samples were in 100 mM potassium phosphate at pH 7.4. B) Soret-excited rR spectra of CO complexes of a) coproheme:Sa decarboxylase, b) heme b:Sa decarboxylase, c) WT DaCld, and d) DaCld(R183Q). Both DaCld enzymes contain heme b. Spectra of CO samples in 100 mM potassium phosphate at pH 6.8 were acquired with 413.1 nm excitation and 2 mW power at the sample. Original spectra, black; fit spectra, red; component bands making up the fit spectra, gray.