Abstract
We recently reported the radiosynthesis and in vitro evaluation of [18F]-2-(4-bromo-2,5-dimethoxyphenyl)-N-(2-(2-fluoroethoxy)benzyl)ethanamine, ([18F]FECIMBI-36) or ([18F]1), an agonist radioligand for 5HT2A/2C receptors in postmortem samples of human brain. Herein we describe the in vivo evaluation of [18F]FECIMBI-36 in vervet /African green monkeys by PET imaging. PET images show that [18F]FECIMBI-36 penetrates the blood-brain barrier and a low retention of radioactivity is observed in monkey brain. Although the time activity curves indicate a somehow heterogeneous distribution of the radioligand in the brain, the low level of [18F]FECIMBI-36 in brain may limit the use of this tracer for quantification of 5-HT2A/2C receptors by PET.
Keywords: 5-HT, 5-HT2A/2CR, agonist, PET, radiotracer
Graphical abstract
Serotonin 2A receptors (5-HT2Rs) are a class of excitatory 5-HT receptor, belonging to the superfamily of G protein coupled receptors (GPCRs).1–4 These receptors comprise of 3 subtypes namely 5-HT2AR, 5-HT2BR and 5-HT2CR. Among these, 5-HT2AR is the most abundant 5-HT receptor in the human brain and it is involved in the pathophysiology of a variety of neuropsychiatric and neurodegenerative diseases and in the aging of brain.5–9 Agonists acting at 5-HT2AR have been evaluated in the treatment of neuropsychiatric disorders. 5-HT2Rs exist in an active high affinity agonist binding state and in an inactive state, in which the receptor binds to agonists with low affinity.2–4 Due to the large receptor density and heterogeneous distribution in brain, 5-HT2AR is an important target for brain imaging with PET.10–14 Measuring the agonist binding to the functional high affinity state by PET would enable determination of the involvement of 5HT2R in the pathology of neuropsychiatric diseases. PET imaging using agonist radioligands can also lead to diagnosis and aid the development of new agonist medications via occupancy measurement studies. The antagonist PET ligands developed so far are not proven successful for the quantification of high affinity confirmation of 5-HT2ARs.12–14 [11F]CIMBI-5 and [11F]CIMBI-36, analogues of dimethoxyphenylethan-1-amine, are the two 5-HT2A/2C agonist radiotracers tested in vivo in pigs and baboons.15–20 So far, and only [11F]CIMBI-36 is currently being investigated in human.21, 22 Although [11F]CIMBI-5 and [11F]CIMBI-36 show proof of concept of in vivo quantification of 5-HT2AR by PET, slow washout in cortical regions, low cortex to cerebellum binding ratios and relatively short half life of [11F] may limit their widespread use in clinical imaging studies. In view of circumventing these shortcomings, we recently developed FECIMBI-36 (1), a high affinity 5-HT2A/2CR agonist ligand that can be radiolabelled with [18F].23 Compound 1 exhibits high affinity to 5-HT2AR (Ki = 1 nM), 5-HT2BR (Ki = 2.8 nM) and 5-HT2CR (Ki = 1.7 nM).23 FECIMBI-36 did not show significant affinity for any other brain receptors and biogenetic amines.23 Functional studies reveal that FECIMBI-36 has an Emax of 63.4% for 5-HT2AR, 18.6% for 5-HT2BR and 100% for 5-HT2CR.23 In vitro autoradiography experiments demonstrate that [18F]FECIMBI-36 labels 5-HT2AR and 5-HT2CR receptors in postmortem human brain sections.23 Herein we describe the results of our in vivo PET imaging results of [18F]FECIMBI-36 in vervet monkey.
Synthesis of radiolabeling precursor, reference standard FECIMBI-36 and [18F]FECIMBI-36 were achieved by our recently reported method.23 In short, radiosynthesis of [18F]FECIMBI-36 was achieved by a one-pot two-step procedure by reacting tert-butoxycarbonyl (Boc) protected precursor with [18F]fluoroethyl tosylate ([18F]FEOTs) in presence of Cs2CO3, followed by removal of Boc group in 25±5% radiochemical yield and > 95% chemical and radiochemical purities. PET imaging was performed in fasted adult male vervet/ African green monkey (Chlorocebus aethiops sabaeus) monkey under anesthetic conditions.24
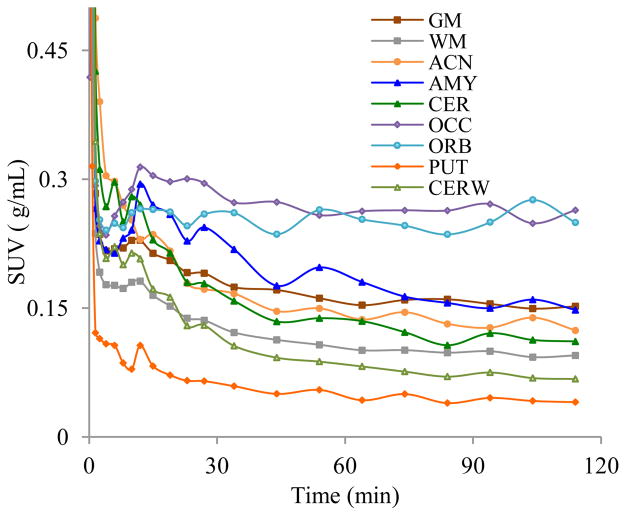
PET images show that although [18F]FECIMBI-36 penetrates the blood-brain barrier (BBB), as indicated by Figure 2, the amount of radioligand accumulated in brain is low. Regional time activity curves (TACs) are shown in Figure 3. TACs show a somehow heterogeneous distribution of [18F[CEIMBI-36 in monkey brain despite low SUV (~0.3 g/mL) in brain. The highest accumulation of radioactivity was found in cortical regions, followed by cerebellum and hippocampus. Among the considered regions of interest, thalamus and putamen show the lowest activity of the radiotracer with relatively rapid washout. The TAC based binding ratios of occipital cortex, orbital cortex, parietal cortex, frontal cortex, amygdala, anterior cingulate, hippocampus, cerebellum, thalamus, caudate, to putamen were 6.5, 6.2, 4.9, 4.5, 3.7, 3.8, 2.5, 2.6, 1.7 and 1.6 respectively at 115 minutes. PET images also show binding of the tracer to white matters in brain. The ratio of cerebral and cerebellar grey to white matters based on TAC analyses were ~1.6 at 115 min.
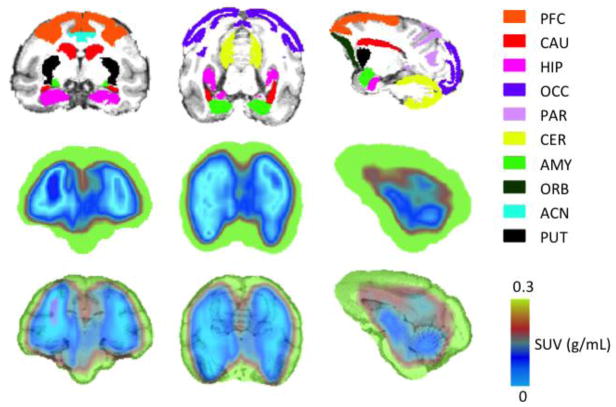
Figure 2.
Ten key brain regions labels in magnetic resonance imaging (MRI) template (Row 1), PET standard uptake value (SUV) (using the sum of radioactivity over a 120 minute scan) (Row 2), and MRI-PET SUV co-registered images (Row 3) of [18F]FECIMBI-36 in vervet monkey brain; Column 1: Axial; Column 2: Coronal; Column 3: Sagittal. (ACN: anterior cingulate; AMY: amygdala; CAU: caudate; CER: cerebellar grey matter; PFC: prefrontal cortex; HIP: hippocampus; OCC: occipital cortex; ORB: orbital cortex; PAR: parietal cortex; PUT: putamen)
Figure 3.
TACs of [18F]FECIMBI-36 in vervet monkey brain (ACN: anterior cingulate; AMY: amygdala; CER: cerebellar grey matter; CERW: cerebellar white matter; OCC: occipital cortex; ORB: orbital cortex; GM: total cerebral grey matter; PUT: putamen; WM: Cerebral white matter).
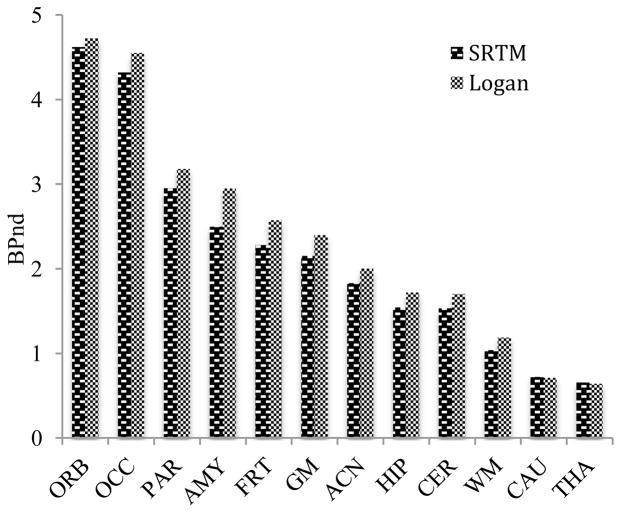
Values of [18F]FECIMBI-36 distribution volume were calculated in the different regions using graphical analysis (Logan plot)25 and an arterial input function, which was not corrected for the presence of radiotracer metabolites (values of distribution volume therefore are here referred to as VT*). Such values are several fold lower than those for typical brain PET ligands, potentially due to low retention or low delivery of radiotracer in brain. Binding potential (BPND) values of [18F]FECIMBI-36 were calculated using the simplified reference tissue model (SRTM)26 and graphical analysis (reference Logan plot)27 with putamen as reference region (Figure 4). Both methods show comparable BPND estimates. Cortical regions show higher BPND values than other regions and the results are in agreement with analyses based on SUV. The PET imaging result in monkeys shown above using [18F]FECIMBI-36 is consistent with the recently reported PET imaging results in pigs regarding low uptake in brain.28 Low brain uptake of [18F]FECIMBI-36 may be partly due to its relatively higher lipophilicity (ClogP = 4.0)23 and low proportion of high affinity agonist binding site of 5-HT2AR in monkey brain.
Figure 4.
Binding potential (BPND) of [18F]FECIMBI-36 in vervet monkey brain regions.
In summary, PET studies in anesthetized monkey show that [18F]FECIMBI-36 penetrates the BBB and exhibits binding in 5-HT2AR enriched regions. Although the proof of concept of in vivo PET imaging using [18F]FECIMBI-36 is demonstrated in monkey, the radioligand showed low brain uptake and VT* values, and thus may not be ideal for further in vivo quantification in brain. Therefore, we pursue more suitable analogues of dimethoxyphenylethan-1-amine or related small molecules to develop successful PET ligands for further investigations.
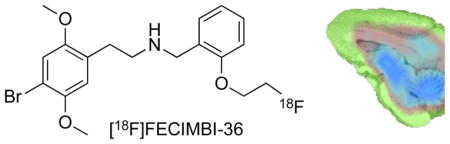
Figure 1.
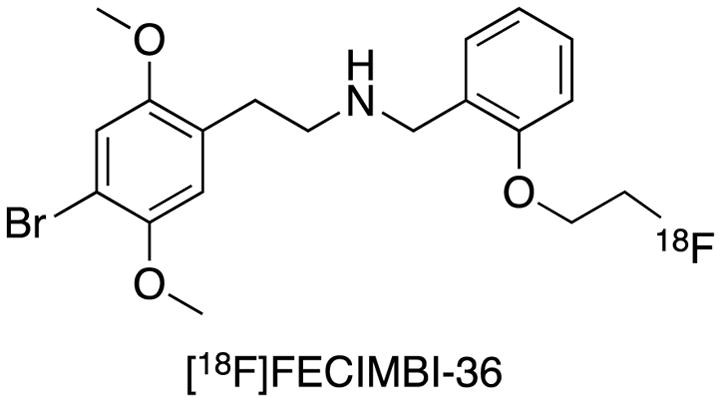
Chemical structure of [18F]FECIMBI-36
Acknowledgments
This work was supported by National Institute of Health grants DA041670 (JP), and OD010965 (JRK). We would like to acknowledge the Translational Imaging Program and the Non-human Primate Signature Program of the Wake Forest Clinical and Translational Science Institute (WF CTSI), which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001420
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Glennon RA, Dukat M, Westkaemper RB. Am Coll Neurophyscopharmacol. 2008;04:11. (2000-01-01) [Google Scholar]
- 2.Nichols DE, Nichols CD. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 3.Mueller C, Jacobs B. Handbook of the Behavioral Neurobiology of Serotonin. Amsterdam: Academic Press/Elsevier; 2010. [Google Scholar]
- 4.Palacios JB. Brain Res. 2016;1645:46–9. doi: 10.1016/j.brainres.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 5.Aznar S, Hervig M-S. Neurosci Biobehav Rev. 2016;64:63–82. doi: 10.1016/j.neubiorev.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Yasue I, Matsunaga S, Kishi T, Fujita K, Iwata N. J Alzh Dis. 2015;50(3):733–40. doi: 10.3233/JAD-150818. [DOI] [PubMed] [Google Scholar]
- 7.Zhang G, Stackman RW., Jr Front Pharmacol. 2015;6:225. doi: 10.3389/fphar.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mestre TA, Zurowski M, Fox SH. Expert Opin Investig Drugs. 2013;4:411–21. doi: 10.1517/13543784.2013.769957. [DOI] [PubMed] [Google Scholar]
- 9.Jin C, Xu W, Yuan J, Wang G, Cheng Z. Neurol Res. 2013;35(1):7–14. doi: 10.1179/1743132812Y.0000000111. [DOI] [PubMed] [Google Scholar]
- 10.Herth MM, Knudsen GM. J Labelled Comp Radiopharm. 2015;58(7):265–73. doi: 10.1002/jlcr.3288. [DOI] [PubMed] [Google Scholar]
- 11.Tyacke RJ, Nutt DJ. Synapse. 2015;69(10):505–11. doi: 10.1002/syn.21835. [DOI] [PubMed] [Google Scholar]
- 12.Kumar JSD, Mann JJ. Cent Nerv Syst Agents Med Chem. 2014;14(2):96–112. doi: 10.2174/1871524914666141030124316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saulin A, Savli M, Lanzenberger R. Amino Acids. 2012;42(6):2039–57. doi: 10.1007/s00726-011-1078-9. [DOI] [PubMed] [Google Scholar]
- 14.Paterson LM, Kornum BR, Nutt DJ, Pike VW, Knudsen GM. Med Res Rev. 2013;33(1):54–111. doi: 10.1002/med.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pigott A, Frescas S, McCorvy JD, Huang XP, Roth BL, Nichols DE. Beilstein J Org Chem. 2012;8:1705–9. doi: 10.3762/bjoc.8.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. Eur J Nucl Med Mol Imaging. 2011;38(4):681–693. doi: 10.1007/s00259-010-1686-8. [DOI] [PubMed] [Google Scholar]
- 17.Ettrup A, Palner M, Gillings N, Santini MA, Hansen M, Kornum BR, Rasmussen LK, Nagren K, Madsen J, Begtrup M, Knudsen GM. J Nucl Med. 2010;51(11):1763–1770. doi: 10.2967/jnumed.109.074021. [DOI] [PubMed] [Google Scholar]
- 18.Ettrup A, Holm S, Hansen M, Wasim M, Santini MA, Palner M, Madsen J, Svarer C, Kristensen JL, Knudsen GM. Mol Imaging Biol. 2013;15(4):376–383. doi: 10.1007/s11307-012-0609-4. [DOI] [PubMed] [Google Scholar]
- 19.Finnema SJ1, Stepanov V, Ettrup A, Nakao R, Amini N, Svedberg M, Lehmann C, Hansen M, Knudsen GM, Halldin C. Neuroimage. 2014;1–84:342–53. doi: 10.1016/j.neuroimage.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 20.Jørgensen LM, Weikop P, Villadsen J, Visnapuu AT, Ettrup S, Hansen HD, Baandrup AO, Andersen FL, Bjarkam CR, Thomsen C, Jespersen B, Knudsen GM. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16629483. pii 0271678X16629483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ettrup A, Bang S-C, McMahon 1B, Lehel S, Dyssegaard A, Skibsted AW, Jørgensen LM, Hansen M, Baandrup AO, Bache S, Svarer 1C, Kristensen JL, Gillings N, Madsen J, Knudsen GM. J Cer Blood Flow Metab. 2014;34:1188–1196. doi: 10.1038/jcbfm.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ettrup A, Svarer C, McMahon B, da Cunha-Bang S, Lehel S, Møller K, Dyssegaard A, Ganz M, Beliveau V, Jørgensen LM, Gillings N, Knudsen GM. Neuroimage. 2016;130:167–74. doi: 10.1016/j.neuroimage.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Prabhakaran J, Underwood MD, Kumar JSD, Simpson NR, Kassir SA, Bakalian MJ, Mann JJ, Arango V. Bioorg Med Chem Lett. 2015;25(18):3933–6. doi: 10.1016/j.bmcl.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.PET imaging of [18F]FECIMBI-36 in monkey: PET scan was performed in vervet monkey (7.76 kg) using a GE 64-slice PET/CT Discovery VCT Scanner (General Electric Medical Systems, Milwaukee, WI, USA) under anesthesia (1.5–2.0% isoflurane). An intravenous line was used for radiotracer injection and to maintain an infusion with 0.9% NaCl throughout the experiment. An arterial line was placed for obtaining arterial samples for calculation of the input function. After a 10 min transmission scan, [18F]FECIMBI-36 was injected (2.65 mCi) as intravenous bolus over 30 seconds, and emission data were collected for 120 min in 3-dimensional mode. Arterial plasma samples were taken manually at 2, 4, 6, 8, 12, 20, 40, 80, 100 and 120 minutes after radiotracer injection. Plasma samples were analyzed using our established procedures to measure the total radiotracer radioactivity in arterial plasma over time.29 Image analysis was performed in MATLAB 2012b (The Mathworks, Natick, MA, USA). Registrations from PET to magnetic resonance image (MRI) and MRI to standard space were performed with FMRIB Software Library (FSL) linear registration module FLIRT.30 Time activity curves (TACs) were then extracted as the mean PET values within each region of interest based on the INIA Primate atlas.31
- 25.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley S. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 26.Lammertsma AA, Hume SP. Neuroimage. 1996;4(3):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 27.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding DL, Alexoff YS. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Herth MM, Petersen IN, Hansen HD, Hansen M, Ettrup A, Jensen AA, Lehel S, Dyssegaard A, Gillings N, Knudsen GM, Kristensen JL. Nucl Med Biol. 2016;43(8):455–62. doi: 10.1016/j.nucmedbio.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Kumar JSD, Majo VJ, Hsiung S–C, Millak MS, Liu K–P, Tamir H, Prabhakaran J, Simpson NR, Van Heertum RL, Mann JJ, Parsey RV. J Med Chem. 2006;49(1):125–34. doi: 10.1021/jm050725j. [DOI] [PubMed] [Google Scholar]
- 30.Jenkinson M, Banniste P, Brady M, Smith S. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 31.Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, Pfefferbaum A. Front Neuroinform. 2012;6:27. doi: 10.3389/fninf.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]