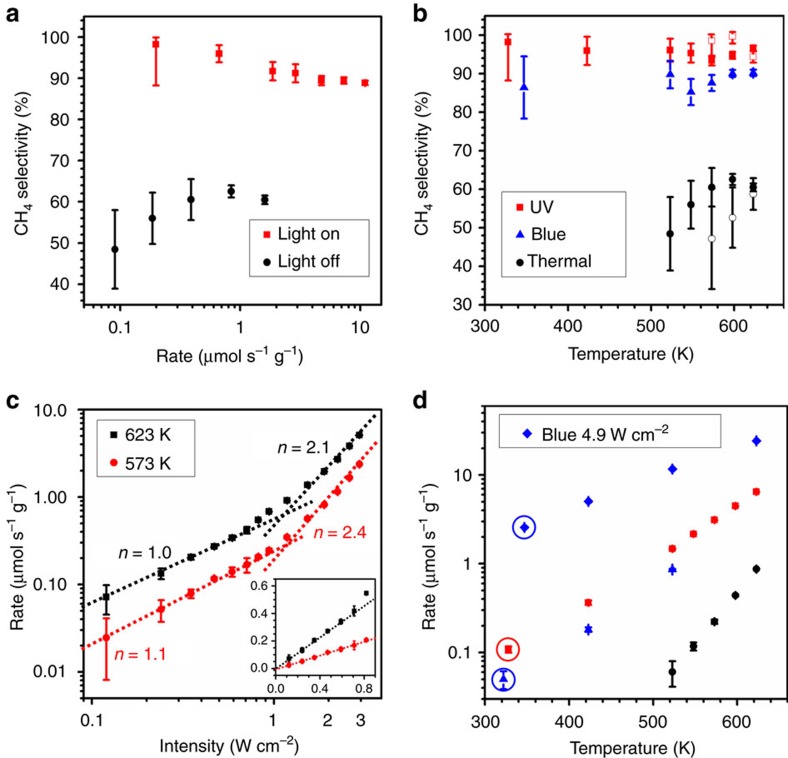
Figure 2. Product selectivity and reaction rates on the rhodium photocatalyst.
(a) Selectivity towards CH4 as a function of overall reaction rates in dark (black circles) and under ultraviolet light at 3 W cm−2 (red squares). (b) Selectivity towards CH4 of the thermo- (black circles) and photocatalytic reactions under ultraviolet (365 nm, red squares) and blue (460 nm, blue triangles) illumination as a function of temperature under H2-rich (CO2:H2=1:5.5, solid symbols) and H2-deficient (CO2:H2=1:3.1, open symbols) conditions. The photoreaction rates are calculated by subtracting the thermocatalytic reaction rates from overall reaction rates at the same temperature. The photoreactions under ultraviolet light show higher selectivity towards CH4 than under blue light, which are both much higher than that of the thermocatalytic reaction. (c) Rates of CH4 photo-production as a function of ultraviolet light intensity at 623 (black squares) and 573 K (red circles). The intensity-dependent reaction rates show a linear to super-linear transition with increasing light intensity. The inset shows the intensity-dependent reaction rates in the linear region. (d) Overall, CH4 production rates in dark (black circles) and under ultraviolet (red squares, 3 W cm−2) and blue (blue triangles, 2.4 W cm−2) LEDs with the same photon flux, and with twice the blue photon flux (blue diamonds, 4.9 W cm−2). Ultraviolet light is more efficient at enhancing the reaction rates than blue light with the same photon flux. Circled points show the unheated steady-state temperatures and reaction rates. Error bars represent the s.d. of measurements by the mass spectrometer.