Abstract
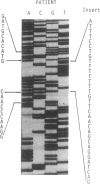
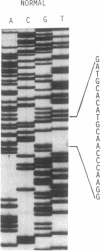
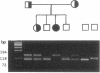
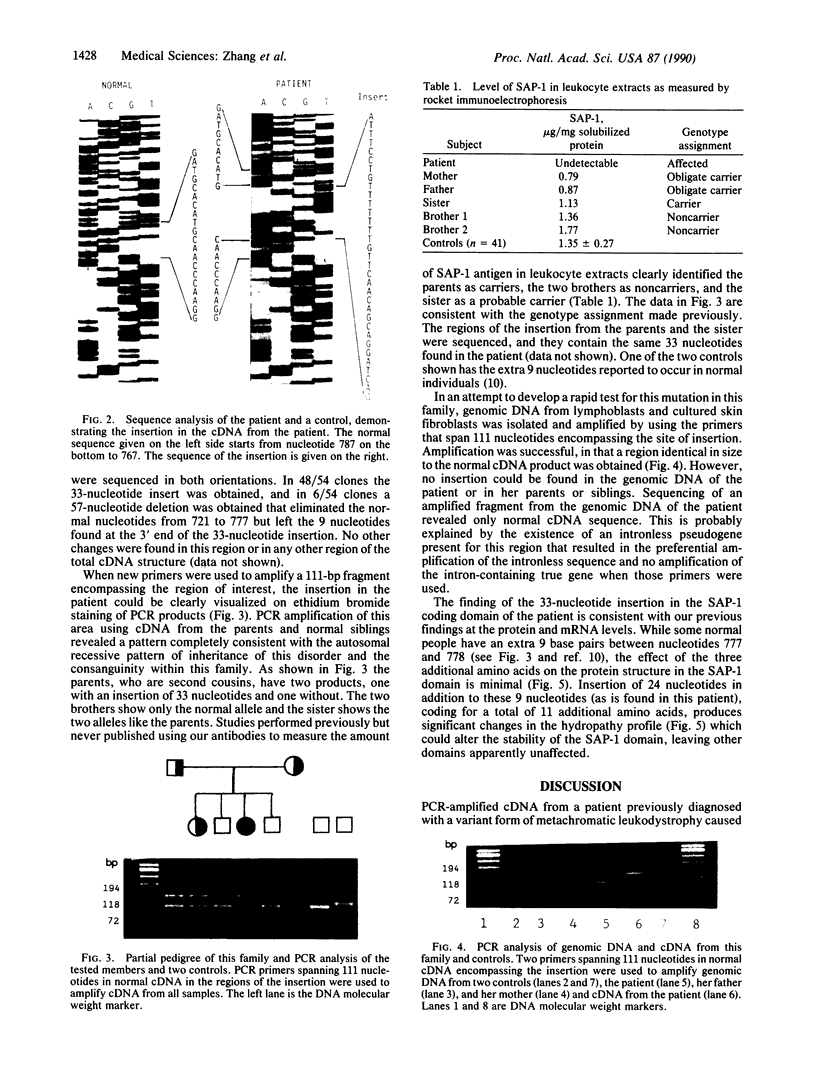
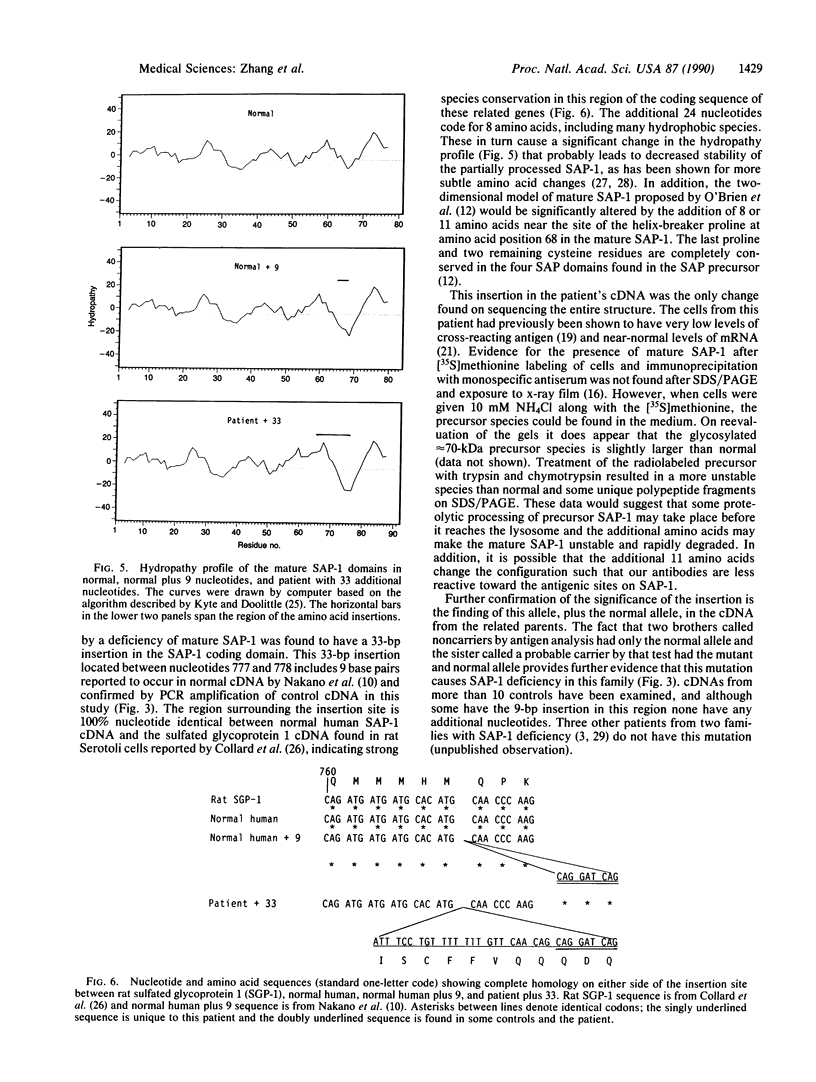
The lysosomal catabolism of sulfatide requires arylsulfatase A and a specific sphingolipid activator protein, SAP-1. While most patients with metachromatic leukodystrophy have mutations in the gene for arylsulfatase A, some patients have deficient SAP-1, as determined by immunological techniques. We now describe the molecular findings in a patient who died at 22 years of age with SAP-1 deficiency. The DNA polymerase chain reaction was used to amplify regions of cDNA which were subcloned in M13 phage DNA and sequenced by the dideoxy chain-termination method. The patient was found to have a 33-base-pair insertion between nucleotides 777 and 778 (numbered from the A of the ATG initiation codon). No other changes were found in the coding sequence of the cDNA from this patient. At the site of the insertion some normal people have an additional 9 base pairs, which correspond to the last 9 nucleotides at the 3' end of the insertion. The cDNAs from the second-cousin parents were amplified and sequenced, and in both two alleles were identified, one with the 33-base-pair insertion and one with no insertion. Two brothers were found to have only the normal alleles and a sister was found to have the 33-base-pair insertion and a normal allele. The findings confirm studies performed on leukocyte extracts demonstrating normal antigen levels in the two brothers and a lower level in the sister. The presence of 11 additional amino acids in the coding region of mature SAP-1 in this patient causes significant changes in the hydropathy profile compatible with the previous findings at the protein level.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christomanou H., Aignesberger A., Linke R. P. Immunochemical characterization of two activator proteins stimulating enzymic sphingomyelin degradation in vitro. Absence of one of them in a human Gaucher disease variant. Biol Chem Hoppe Seyler. 1986 Sep;367(9):879–890. doi: 10.1515/bchm3.1986.367.2.879. [DOI] [PubMed] [Google Scholar]
- Collard M. W., Sylvester S. R., Tsuruta J. K., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 1 secreted by rat Sertoli cells: sequence similarity with the 70-kilodalton precursor to sulfatide/GM1 activator. Biochemistry. 1988 Jun 14;27(12):4557–4564. doi: 10.1021/bi00412a050. [DOI] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. AB variant of infantile GM2 gangliosidosis: deficiency of a factor necessary for stimulation of hexosaminidase A-catalyzed degradation of ganglioside GM2 and glycolipid GA2. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3979–3983. doi: 10.1073/pnas.75.8.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewji N. N., Wenger D. A., O'Brien J. S. Nucleotide sequence of cloned cDNA for human sphingolipid activator protein 1 precursor. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8652–8656. doi: 10.1073/pnas.84.23.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewji N., Wenger D., Fujibayashi S., Donoviel M., Esch F., Hill F., O'Brien J. S. Molecular cloning of the sphingolipid activator protein-1 (SAP-1), the sulfatide sulfatase activator. Biochem Biophys Res Commun. 1986 Jan 29;134(2):989–994. doi: 10.1016/s0006-291x(86)80518-6. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S., Inui K., Wenger D. A. Activator protein-deficient metachromatic leukodystrophy: diagnosis in leukocytes using immunologic methods. J Pediatr. 1984 May;104(5):739–742. doi: 10.1016/s0022-3476(84)80957-9. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S., Kao F. T., Jones C., Morse H., Law M., Wenger D. A. Assignment of the gene for human sphingolipid activator protein-2 (SAP-2) to chromosome 10. Am J Hum Genet. 1985 Jul;37(4):741–748. [PMC free article] [PubMed] [Google Scholar]
- Fujibayashi S., Wenger D. A. Biosynthesis of the sulfatide/GM1 activator protein (SAP-1) in control and mutant cultured skin fibroblasts. Biochim Biophys Acta. 1986 Feb 28;875(3):554–562. doi: 10.1016/0005-2760(86)90077-9. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S., Wenger D. A. Synthesis and processing of sphingolipid activator protein-2 (SAP-2) in cultured human fibroblasts. J Biol Chem. 1986 Nov 15;261(32):15339–15343. [PubMed] [Google Scholar]
- Fürst W., Machleidt W., Sandhoff K. The precursor of sulfatide activator protein is processed to three different proteins. Biol Chem Hoppe Seyler. 1988 May;369(5):317–328. doi: 10.1515/bchm3.1988.369.1.317. [DOI] [PubMed] [Google Scholar]
- Hahn A. F., Gordon B. A., Hinton G. G., Gilbert J. J. A variant form of metachromatic leukodystrophy without arylsulfatase deficiency. Ann Neurol. 1982 Jul;12(1):33–36. doi: 10.1002/ana.410120106. [DOI] [PubMed] [Google Scholar]
- Inui K., Emmett M., Wenger D. A. Immunological evidence for deficiency in an activator protein for sulfatide sulfatase in a variant form of metachromatic leukodystrophy. Proc Natl Acad Sci U S A. 1983 May;80(10):3074–3077. doi: 10.1073/pnas.80.10.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui K., Kao F. T., Fujibayashi S., Jones C., Morse H. G., Law M. L., Wenger D. A. The gene coding for a sphingolipid activator protein, SAP-1, is on human chromosome 10. Hum Genet. 1985;69(3):197–200. doi: 10.1007/BF00293023. [DOI] [PubMed] [Google Scholar]
- Inui K., Wenger D. A. Biochemical, immunological, and structural studies on a sphingolipid activator protein (SAP-1). Arch Biochem Biophys. 1984 Sep;233(2):556–564. doi: 10.1016/0003-9861(84)90479-x. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt T., Christomanou H., Braunitzer G. Complete amino-acid sequence of the naturally occurring A2 activator protein for enzymic sphingomyelin degradation: identity to the sulfatide activator protein (SAP-1). Biol Chem Hoppe Seyler. 1988 Dec;369(12):1361–1365. doi: 10.1515/bchm3.1988.369.2.1361. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Morimoto S., Martin B. M., Kishimoto Y., O'Brien J. S. Saposin D: a sphingomyelinase activator. Biochem Biophys Res Commun. 1988 Oct 14;156(1):403–410. doi: 10.1016/s0006-291x(88)80855-6. [DOI] [PubMed] [Google Scholar]
- Morimoto S., Martin B. M., Yamamoto Y., Kretz K. A., O'Brien J. S., Kishimoto Y. Saposin A: second cerebrosidase activator protein. Proc Natl Acad Sci U S A. 1989 May;86(9):3389–3393. doi: 10.1073/pnas.86.9.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Sandhoff K., Stümper J., Christomanou H., Suzuki K. Structure of full-length cDNA coding for sulfatide activator, a Co-beta-glucosidase and two other homologous proteins: two alternate forms of the sulfatide activator. J Biochem. 1989 Feb;105(2):152–154. doi: 10.1093/oxfordjournals.jbchem.a122629. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Kretz K. A., Dewji N., Wenger D. A., Esch F., Fluharty A. L. Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science. 1988 Aug 26;241(4869):1098–1101. doi: 10.1126/science.2842863. [DOI] [PubMed] [Google Scholar]
- Ohno K., Suzuki K. Multiple abnormal beta-hexosaminidase alpha chain mRNAs in a compound-heterozygous Ashkenazi Jewish patient with Tay-Sachs disease. J Biol Chem. 1988 Dec 5;263(34):18563–18567. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano A., Radin N. S., Johnson L. L., Tarr G. E. The activator protein for glucosylceramide beta-glucosidase from guinea pig liver. Improved isolation method and complete amino acid sequence. J Biol Chem. 1988 Dec 25;263(36):19597–19601. [PubMed] [Google Scholar]
- Shapiro L. J., Aleck K. A., Kaback M. M., Itabashi H., Desnick R. J., Brand N., Stevens R. L., Fluharty A. L., Kihara H. Metachromatic leukodystrophy without arylsulfatase A deficiency. Pediatr Res. 1979 Oct;13(10):1179–1181. doi: 10.1203/00006450-197910000-00021. [DOI] [PubMed] [Google Scholar]
- Valerio D., Dekker B. M., Duyvesteyn M. G., van der Voorn L., Berkvens T. M., van Ormondt H., van der Eb A. J. One adenosine deaminase allele in a patient with severe combined immunodeficiency contains a point mutation abolishing enzyme activity. EMBO J. 1986 Jan;5(1):113–119. doi: 10.1002/j.1460-2075.1986.tb04184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger D. A., DeGala G., Williams C., Taylor H. A., Stevenson R. E., Pruitt J. R., Miller J., Garen P. D., Balentine J. D. Clinical, pathological, and biochemical studies on an infantile case of sulfatide/GM1 activator protein deficiency. Am J Med Genet. 1989 Jun;33(2):255–265. doi: 10.1002/ajmg.1320330223. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Grandchamp B., Beaumont C., Picat C., Nordmann Y. Uroporphyrinogen decarboxylase structural mutant (Gly281----Glu) in a case of porphyria. Science. 1986 Nov 7;234(4777):732–734. doi: 10.1126/science.3775362. [DOI] [PubMed] [Google Scholar]