Abstract
Background
The study aimed at enumerating, identifying and categorizing the endophytic cultivable bacterial community in selected salad vegetables (carrot, cucumber, tomato and onion). Vegetable samples were collected from markets of two vegetable hot spot growing areas, during two different crop harvest seasons. Crude and diluted vegetable extracts were plated and the population of endophytic bacteria was assessed based on morphologically distinguishable colonies. The bacterial isolates were identified by growth in selective media, biochemical tests and 16S rRNA gene sequencing.
Results
The endophytic population was found to be comparably higher in cucumber and tomato in both of the sampling locations, whereas lower in carrot and onion. Bacterial isolates belonged to 5 classes covering 46 distinct species belonging to 19 genera. Human opportunistic pathogens were predominant in carrot and onion, whereas plant beneficial bacteria dominated in cucumber and tomato. Out of the 104 isolates, 16.25% are human pathogens and 26.5% are human opportunistic pathogens.
Conclusions
Existence of a high population of plant beneficial bacteria was found to have suppressed the population of plant and human pathogens. There is a greater potential to study the native endophytic plant beneficial bacteria for developing them as biocontrol agents against human pathogens that are harboured by plants.
Electronic supplementary material
The online version of this article (doi:10.1186/s12866-017-0974-x) contains supplementary material, which is available to authorized users.
Keywords: Bacteriome, Endophyte, Human pathogenic, Plant beneficial, Salad, Vegetables
Background
Fresh vegetables are considered as the essential components of healthy diet of people and the consumption of vegetables in the form of salads has increased in many parts of the world, including India. In contrast to the potential health benefits of fresh vegetables, a concern about the safety and the quality of vegetables has also raised due to outbreaks of infectious diseases reported from by Center for Disease Control and Prevention (CDC), US Food and Drug Administration (FDA), World Health Organization (WHO) and Center for Science in the Public Interest (CSPI). These changes are mainly due to change in the ecology of human pathogens to persist in non-host environments.
Since the contamination of salad vegetables with human pathogenic bacteria has reached concerning proportions in recent years, which has been evidenced by reports of various public health agencies through enhanced epidemiological and surveillance techniques, the raw vegetables are undoubtedly the portable source of infectious microorganisms, which has been revealed by numerous outbreaks associated with the consumption of salad vegetables [1, 2].
In general, fresh vegetables are known to harbour large bacterial populations [3], which may be of plant endophytes, plant pathogenic and human pathogenic in nature. The most important features of plant host colonization is by the adaptation of pathogens to the host defence response, physiology, immunity, native microflora, physical barriers, mobility and temperature. The pathogenic or non-pathogenic bacteria have several points of opportunities to contaminate fresh vegetables from the field through the time of consumption [4]. However, the route cause for the contamination of these vegetables, survival rate of endophytic bacterial communities, survival rate of pathogens which may be for plant or/and human, their interaction strategies, survival mechanisms are still under exploration.
Since the fresh vegetables in the form of salads are consumed raw, the pathogens present in it lead to widespread disease outbreaks. The non-pathogenic microbes associated with plants as a commensal or pathogen may leads to allergies which is still undeterminable due to change in the interaction strategies of microbes with the endophytic bacterial community and the plant host [5]. There are several reported outbreaks related to salad vegetables from the past decades to the present. Recently, Listeriosis outbreak was reported by CDC on January 28, 2016 from the consumption of mixed salad vegetables; E. coli 0157 outbreak was reported by USA Today newsletter related to salad vegetables in costco chicken; multistate Salmonella outbreak was linked to cucumbers in 2015 and thus the outbreaks are expanding.
In order to step on to the control of these outbreaks, detail reports of the endophytic bacterial community of vegetables used for salads, survival rate of pathogens in non-host environment etc. have to be identified. The present study was undertaken to find the endophytic bacterial community of the most commonly used South Indian salad vegetables like carrot, cucumber onion and tomato sold in vegetable markets. The study was undertaken by collecting the vegetable samples from two different vegetable growing hot spots of Tamil Nadu, India. Further, the endophytes were classified based on the evolutionary relationship to identify the predominant endophytic taxonomical group in salad vegetables and further classified based on their specific known functions such as human pathogens, human commensals, plant pathogens, plant commensals and environmentally beneficial bacteria.
Methods
Sampling method and surface sanitization
Fresh, damage-free whole salad vegetable samples (carrot, cucumber, onion and tomato) were purchased from two different local markets in Tamil Nadu, India (Hosur and Salem) during April and October of 2015. In Tamil Nadu, these two places are situated in the North Western agro climatic zone, but they differ in their soil type. The vegetable samples were collected in sterile plastic bags and transported to the laboratory as soon as possible. Samples were stored at 4 °C and tested within 48 h. Carrot, cucumber and tomato samples were washed with sterile deionized water and the external surface was scrubbed with an alcoholic solution of iodine (2%), and allowed to air dry inside a laminar airflow cabinet. The outer layer of onion was peeled and washed with deionized water and the external surface was scrubbed with an alcoholic solution of iodine (2%). Sterilization efficacy was evaluated by cutting the scrubbed external surface with sterile scalpel blade and placing directly on the surface of nutrient agar medium. The method chosen for sterilization, treatment with alcoholic solution of 2% iodine and drying under UV light on each side of vegetables, proved to be effective in killing the surface associated bacteria. For every sample batch, two samples of each vegetable were randomly chosen following surface sterilization and placing the external surface on nutrient agar plate. After 24 h of incubation at 37 °C, none of the tested vegetables showed bacterial growth.
Endophytic bacteriological analysis of salad vegetables
Twenty five grams of each salad vegetable sample was weighed aseptically and homogenized by blending in 225 mL of sterile buffered peptone water using commercial blender. One millilitre of each homogenate was mixed with 9 mL of sterile 1% buffered peptone water in a sterile test tube, labelled 1:10 (10-1) dilution and subsequent dilution was done in five other test tubes labelled 10-2, 10-3 and 10-4. The same procedure was repeated for each sample and the blender was cleaned carefully and disinfected in between each samples to prevent cross contamination. For each vegetable, 100 μL of undiluted (crude extract) and diluted (10-2, 10-3, 10-4) samples were plated separately on nutrient agar plates (HiMedia, Mumbai, India) by standard spread plate technique and incubated aerobically at 37 °C for 24 h. All the discrete colonies were counted and expressed as colony forming units per gram (CFU g-1) of vegetable samples. Plating was done in three replications and the colony count was averaged.
Isolation and biochemical characterization of endophytic bacteria
Pure cultures were obtained by streaking isolated single colonies on nutrient agar plate by quadrant streak method and incubated at 37 °C for 24 h. Colonies were presumptively distinguished based on the colony morphology on nutrient agar and selective media including AIA (Aeromonas Isolation Agar), CA (Certemaid Agar), EMBA (Eosin Methylene Blue Agar), MSA (Mannitol Salt Agar), SSA (Salmonella—Shigella Agar) and further tested for Gram’s reaction, M (motility), I (Indole), MR (Methyl Red), VP (Voges – Proskauer), C (Citrate), C (Catalase), O (Oxidase) and N (Nitrate) and incubated at 37 °C for 24 h.
Isolation of DNA from endophytic bacteria
Endophytic bacterial isolates from different salad vegetables were grown on nutrient agar plate and incubated overnight at 37 °C. A single colony was suspended in a micro-centrifuge tube containing 30 μl of sterile nuclease free water and heated at 94 °C for 10 min. The sample was cooled to room temperature and centrifuged at 10,000 rpm for 10 min and the supernatant was directly used as template DNA for PCR.
Molecular identification of endophytes by 16S rRNA gene sequencing
Partial sequencing of the 16S rRNA gene was done by PCR amplification of endophytic bacterial isolates using universal primers. SBBUF (5’-AGAGTTTGATCATGGCTCAG-3’) and SBBUR (5’-TACGGCTACCTTGTTACGAC-3’) primers were used as forward and reverse primers, respectively. PCR amplification was carried out in a thermocycler (Eppendorf, Mastercycler, Hamburg, Germany). The reactions were routinely performed in 20 μl: 2 μl of 30 ng/μl endophytic bacterial DNA, 1 μl of 0.5 μM of each of the opposing amplification primers, 6 μl of nuclease free water and 10 μl of 1X PCR master mix (Ampliquon, Denmark) containing 1.5 mM MgCl2,Tris HCl pH 8.5, (NH4)2SO4, 0.2% Tween 20, 0.4 mM dNTPs, 0.2 U/μl Taq DNA polymerase, inert red dye and stabilizer. PCR amplification program for 16S rRNA primers includes an initial denaturation of template DNA at 94 °C for 10 min followed by 30 cycles with a denaturation step at 94 °C for 1 min, annealing at 49 °C for 1 min, and extension at 72 °C for 1 min, and final extension at 72 °C for 7 min. PCR amplified products were separated on 1% agarose gels containing ethidium bromide with molecular weight marker and visualized under a UV light. PCR products were purified and sequenced at Amnion Bioscience Pvt. Ltd. (Bengaluru, India) to determine the diversity and composition of the salad vegetables associated bacterial communities. Sequencing was performed with SBBUF and SBBUR primers using ABI 3730xl genetic analyser. The resulted nucleotide sequences were edited for any overlaps using Chromas Lite software.
Sequence analysis and classification
To ascertain the phylogenetic affiliation of the endophytic bacterial isolates, partial nucleotide sequences were assigned bacterial taxonomic affiliations based on the closest match to sequences available in the GenBank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) using the Basic Local Alignment Search Tool (BLAST) search algorithm. Sequence comparisons were done by constructing a sequence data matrix of 16S rRNA genes of each endophytic bacterial isolate and further classified based on their nature of existence as human pathogen, human opportunistic pathogen, plant pathogen, plant beneficial, plant commensal and environmentally beneficial bacteria.
Phylogenetic trees were constructed based on neighbour-joining [6] and maximum-parsimony method using MEGA software, version 6 [7]. Bootstrap analyses were performed for 1000 replications. The nucleotide positions containing gaps and missed data were eliminated. The 16S rRNA gene sequences determined in the study was deposited in the GenBank sequence library and their accession numbers are indicated on the tree.
Diversity indices of endophytic bacteria
The diversity indices of the endophytic bacterial isolates were calculated by using the PAST program version 2.10 [8].
Results
The results of this study provide insight into the identification of dominant members of the endophytic bacterial communities on the common South Indian salad vegetables such as carrot, cucumber onion and tomato.
The endophytic population was found to be comparably higher in cucumber and tomato in both of the sampling locations, whereas lower in carrot and onion (Fig. 1). The per gram fresh weight population density of endophytic bacterial isolates in carrot ranged from 5.4 × 10-1 (crude extract) to 3.6 × 10-4 (10-4 dilution) CFU g-1, that of fresh cucumber ranged from 5.5 × 10-1 (crude extract) to 4.2 × 10-4 CFU g-1(10-4 dilution), fresh onion from 5.1 × 10-1 (crude extract) to 3.4 × 10-4 CFU g-1 (10-4 dilution) and of fresh tomato from 5.5 × 10-1 (crude extract) to 4.3 × 10-4 CFU g-1(10-4 dilution). The reduction in population density by dilution of vegetable extract was more in onion compared to other vegetables. A total of 104 endophytic bacterial isolates were isolated from four vegetables based on their distinguishable colony morphology (shape, size, colour and margin). The total number of morphologically distinguishable isolates obtained from crude and diluted extracts of tomato collected in two seasons is 23 (Hosur – 16; Salem – 7), cucumber is 31 (Hosur – 21; Salem – 10), carrot is 30 (Hosur – 24; Salem – 6) and onion is 20 (Hosur – 14; Salem – 6). In the case of carrot collected from both locations and tomato from Salem, all the isolates are either from crude extract or 10-2 dilution. The isolates were designated as SBAN (authors name), followed by location of sample (H-Hosur; S – Salem), common name of vegetable (CA or Ca – carrot; Cu – cucumber; O – onion; T – tomato) and the isolate number. Isolates obtained from crude extract and dilutions were numbered in sequential order. Since designation of some of the isolates were found to be overlapping after GenBank submission, they were labelled as 1a, 2a etc for the purpose of differentiation.
Fig. 1.
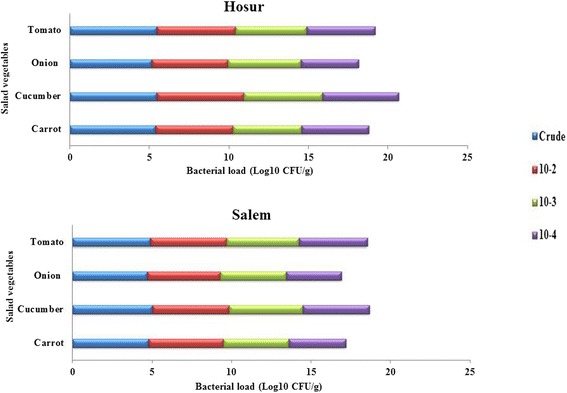
Endophytic bacterial population from internal tissues of salad vegetables
The endophytic bacterial isolates were further characterized by observing their growth and colony morphology on selective media. On AIA media, only one isolate showed growth (SBANHCu11) and it was from Hosur cucumber. On CA media, the isolates SBANHCa1, SBANHCA14, SBANHCu19, SBANHCu20, SBANHCu22, SBANHCu25, SBANCu25a, SBANSCu19, SBANSCu20, SBANSCu22, SBANHO5, SBANHO8 and SBANHO14 exhibited yellowish-green to blue colonies, which represented the presence of Pseudomonas spp. EMB agar screening revealed no isolates with green-metallic sheen, which represents the absence of E. coli. SBANHCA2, SBANHCa7a, SBANHCa10, SBANHCa11, SBANHCa14a, SBANSCu7, SBANHO2, SBANHO15, SBANSO90, SBANHT7, SBANHT9, SBANHT14 and SBANHT15 isolates showed yellowish colonies in MSA media, which indicated the presence of Staphylococcus spp. and on SS agar medium the isolates SBANHCA3, SBANHCa4 and SBANHT11 showed black colour colonies, representing the presence of Salmonella spp.
Further, biochemical characterization of endophytic bacterial isolates was done in which, about 57.7% (n = 60) of the isolates were identified as Gram positive and the remaining 42.3% (n = 44) of the isolates as Gram negative. The existence of rod shaped endophytic bacterial population were about 87.5% (n = 91) and the cocci were about 12.5% (n = 13). In motility test, 79.8% (n = 83) were found to be motile and 20.2% (n = 21) were non-motile. The isolates were also classified based on I, MR, VP, C, catalase, oxidase and nitrate test, and the results are shown in Additional file 1: Table S1(a), (b), (c) and (d).
Molecular characterization of all the 104 endophytic bacterial isolates was carried out by PCR amplification of the genomic DNA using universal bacterial primers for 16S rRNA gene. The expected size of 1.2 kb fragment for each of the 104 isolates was obtained and sequenced. The nucleotide sequences were searched for homology in the NCBI GenBank database using the BLASTn program. The results showed highest homology of > 97 to 100. Based on the 16S rRNA gene sequence identity, bacterial isolates were found belonging to 5 classes namely actinobacteria (11%), bacilli (43%), cocci (4%), betaproteobacteria (3%) and gammaproteobacteria (39%) with 46 distinct species belonging to 19 genera. The data also indicated the existence of more diverse endophytic bacterial communities in tomato and cucumber in both the sampling locations as compared to onion and carrot. Among the various endophytic bacterial populations, the class bacilli was found to be predominant with 21 diverse genera followed by gammaproteobacteria (14 genera), actinobacteria (8 genera), betaproteobacteria (2 genera) and cocci (1 genera). Actinobacteria was found to be distributed high in cucumber compared to other salad vegetables. Distribution of bacilli was high in tomato, whereas cocci were predominantly present in carrot. Betaproteobacteria and gammaproteobacteria were high in cucumber.
The taxonomical classification of endophytic bacterial isolates of salad vegetables observed in the study is presented in Table 1. Phylogenetic analysis was done by constructing the neighbour joining phylogenetic tree of all the 104 endophytic bacterial isolates to explore the evolutionary relationship, and the evolutionary distance was calculated using maximum composite likelihood method using MEGA software, version 6. Figure 2 represents the phylogenetic tree based on 16S rRNA sequences of the isolated bacteria. The overall class distribution of bacterial isolates from all the four vegetables of both of the sampling locations is illustrated in Fig. 3. Sequences were deposited in the GenBank and the accession numbers for each of the bacterial isolate along with details of their pathogen/non-pathogen nature (based on literature search) are presented in Table 2.
Table 1.
Taxonomical classification of the bacterial isolates from salad vegetables
| Isolate ID | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|
| SBANHCA1, SBANHCa5, SBANHCa6, SBANHCa13, SBANHO7 | Proteobacteria | Gammaproteobacteria | Xanthomonadales | Xanthomonadaceae | Stenotrophomonas | maltophilia |
| SBANHCA2, SBANHCa10, SBANHCa11, SBANHO2 | Firmicutes | Coccus | Bacillales | Staphylococcaceae | Staphylococcus | aureus |
| SBANHCA3, SBANHCa4, SBANHT11 | Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Salmonella | enterica |
| SBANHCA7, SBANHCa9 | Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Enterobacter | aerogenes |
| SBANHCa8 | Proteobacteria | Betaproteobacteria | Burkholderiales | Alcaligenaceae | Bordetella | bronchiseptica |
| SBANHCa12, SBANHCA15, SBANHCA17, SBANHCA15a, SBANHCa17a, SBANHO1, SBANHO4, SBANHO12, SBANSO81, SBANHT10, SBANHT12, SBANST3 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | pumilus |
| SBANHCA14, SBANHCa1a | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | aeruginosa |
| SBANHCA16 | Firmicutes | Bacilli | Bacillales | Paenibacillaceae | Paenibacillus | polymyxa |
| SBANHCa16a | Firmicutes | Bacilli | Bacillales | Paenibacillaceae | Paenibacillus | illinoisensis |
| SBANHCa2a | Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Enterobacter | hormaechei |
| SBANHCa7a, SBANHT9, SBANHT14, SBANHT15 SBANHCa14a |
Firmicutes | Bacilli | Bacillales | Staphylococcaceae | Staphylococcus | sciuri |
| SBANSCa3 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | aerophilus |
| SBANSCa4 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | cereus |
| SBANSCa5 | Actinobacteria | Actinobacteria | Actinomycetales | Microbacteriaceae | Microbacterium | oleivorans |
| SBANSCa6 | Actinobacteria | Actinobacteria | Micrococcales | Micrococcaceae | Arthrobacter | nicotianae |
| SBANSCa7, SBANHT4, SBANST8, SBANST12 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | subtilis |
| SBANSCa10, SBANSO82, SBANHT3 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | flexus |
| SBANHCu23a, SBANHCu15, SBANHCu23, SBANHCu16, SBANHCu17, SBANHCu14a, SBANHCu15a, SBANHCu17a, SBANHT1, SBANHT16, SBANHT17 | Proteobacteria | Gammaproteobacteria | Xanthomonadales | Xanthomonadaceae | Stenotrophomonas | rhizophila |
| SBANHCu24 | Actinobacteria | Actinobacteria | Micrococcales | Micrococcaceae | Arthrobacter | mysorens |
| SBANHCu25a, SBANSCu19, SBANHCu19, SBANHCu22, SBANSCu22, | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | xanthomarina |
| SBANHCu10, SBANHT21 | Actinobacteria | Actinobacteria | Micrococcales | Promicromonosporaceae | Cellulosimicrobium | cellulans |
| SBANHCu12 | Actinobacteria | Actinobacteria | Micrococcales | Microbacteriaceae | Microbacterium | schleiferi |
| SBANHCu14 | Proteobacteria | Gammaproteobacteria | Xanthomonadales | Xanthomonadaceae | Xanthomonas | axonopodis |
| SBANHCu20, SBANHO3, SBANHO5, SBANHO8, SBANHO13, SBANHO14 | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | stutzeri |
| SBANHCu21, SBANSCu21 | Proteobacteria | Betaproteobacteria | Burkholderiales | Alcaligenaceae | Achromobacter | xylosoxidans |
| SBANHCu24a | Actinobacteria | Actinobacteria | Micrococcales | Micrococcaceae | Arthrobacter | protophormiae |
| SBANHCu25 | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | indoloxydans |
| SBANHCu11 | Proteobacteria | Gammaproteobacteria | Aeromonadales | Aeromonadaceae | Aeromonas | hydrophila |
| SBANSCu4 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Geobacillus | stearothermophilus |
| SBANSCu7 | Firmicutes | Bacilli | Bacillales | Staphylococcaceae | Staphylococcus | haemolyticus |
| SBANSCu8 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | aerius |
| SBANSCu9, SBANSCu11, SBANST7, SBANST11 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | megaterium |
| SBANSCu10, SBANHCu12a, SBANSO80 | Actinobacteria | Actinobacteria | Micrococcales | Microbacteriaceae | Microbacterium | arborescens |
| SBANSCu20 | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | fluorescens |
| SBANHO6 | Proteobacteria | Gammaproteobacteria | Xanthomonadales | Xanthomonadaceae | Xanthomonas | fuscans |
| SBANHO9 | Firmicutes | Bacilli | Bacillales | Listeriaceae | Listeria | monocytogenes |
| SBANHO11 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | anthracis |
| SBANHO15 | Firmicutes | Bacilli | Bacillales | Staphylococcaceae | Staphylococcus | epidermidis |
| SBANSO90, SBANHT7 | Firmicutes | Bacilli | Bacillales | Staphylococcaceae | Staphylococcus | gallinarum |
| SBANSO54 | Firmicutes | Bacilli | Lactobacillales | Enterococcaceae | Enterococcus | faecium |
| SBANSO83 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | aryabhattai |
| SBANHT8 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Geobacillus | stearothermophilus |
| SBANHT13 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Exiguobacterium | acetylicum |
| SBANHT19 | Actinobacteria | Actinobacteria | Micrococcales | Microbacteriaceae | Microbacterium | testaceum |
| SBANST6 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Terribacillus | saccharophilus |
| SBANST6a | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | tequilensis |
Fig. 2.
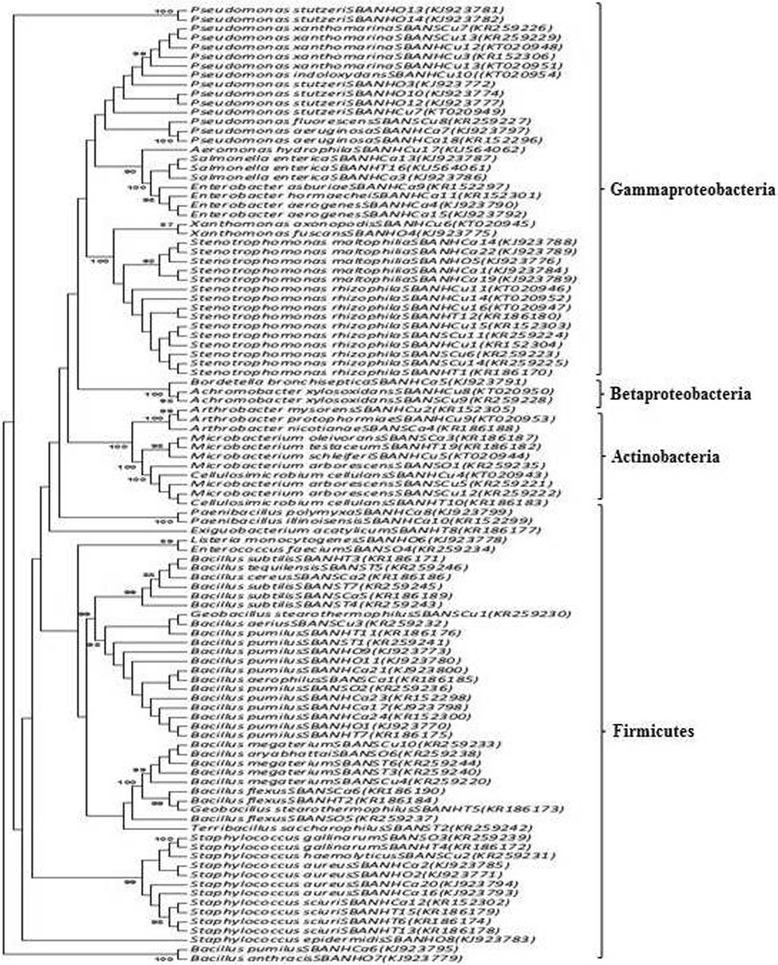
Phylogenetic tree of endophytic bacteria in salad vegetables based on 16S rRNA gene sequences
Fig. 3.
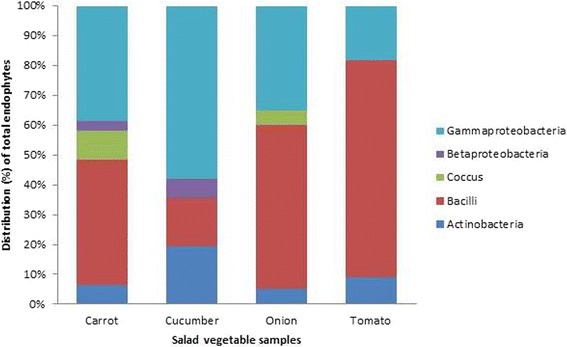
Taxonomical distribution of endophytic bacteria in salad vegetables
Table 2.
Endophytic bacterial isolates from salad vegetables and their known characteristics
| Bacterial isolate | Descriptiona | Source vegetable | Isolate ID | Dilution | GenBank accession no. |
|---|---|---|---|---|---|
| Stenotrophomonas maltophilia | Opportunistic pathogen; associated with conjunctivitis, keratitis, scleritis, dacryrocystitis, cellulitis and endophthalmitis | Carrot, Carrot, Carrot, Carrot, Onion, Tomato |
SBANHCA1, SBANHCa5, SBANHCa6, SBANHCa13, SBANHO7, SBANHT8 |
Crude, Crude, Crude, 10-2, Crude, Crude |
KJ923784, KJ923788, KJ923789, KJ923796, KJ923776, KR186173 |
| Staphylococcus aureus | Opportunistic pathogen; causes food poisoning, skin infection, pneumonia, septicemia, pyogenic infection, toxic shock syndrome and bacteremia | Carrot, Carrot, Carrot, Onion, Tomato, |
SBANHCA2, SBANHCa10, SBANHCa11, SBANHO2, SBANHT3, |
Crude, Crude, 10-2, Crude, Crude, |
KJ923785, KJ923793, KJ923794, KJ923771, KR186184, |
| Terribacillus saccharophilus | Plant growth promoting rhizobacteria | Tomato | SBANST6 | Crude | KR259242 |
| Bacillus tequilensis | Plant growth promoting bacteria; nitrogen fixing | Tomato | SBANST6a | Crude | KR259246 |
| Salmonella enterica | Highly pathogenic; causes food and water borne disease in human and animals; causes gastroenteritis, bacteraemia, enteric fever | Carrot, Carrot |
SBANHCA3, SBANHCa4 | Crude, Crude | KJ923786, KJ923787 |
| Enterobacter aerogenes | Opportunistic pathogen; causes urinary tract infection, bacteraemia, endocarditis, septic arthritis, osteomyelitis and skin/soft tissue infection | Carrot, Carrot |
SBANHCA7, SBANHCA9 | Crude, Crude | KJ923790, KJ923792 |
| Bordetella bronchiseptica | Opportunistic pathogen; causes respiratory tract infection in patients with cystic fibrosis and whooping cough | Carrot | SBANHCa8 | Crude | KJ923791 |
| Bacillus pumilus | Plant growth promoting bacteria; opportunistic pathogen; causes food poisoning and cutaneous infection in human. | Carrot, Carrot, Carrot, Carrot, Carrot, Onion, Onion, Onion, Onion, Tomato, Tomato, Tomato, Tomato, Tomato, Tomato |
SBANHCa12, SBANHCA15, SBANHCa15a, SBANHCA17, SBANHCa17a, SBANHO1, SBANHO4, SBANHO12, SBANSO81, SBANHT1, SBANHT16, SBANHT17, SBANHT21, SBANST8, SBANST12 |
10-2, 10-2, 10-2, 10-2, 10-2, Crude, Crude, 10-4, Crude, Crude, 10-2, 10-3, 10-4, 10-2, 10-2 |
KJ923795, KJ923798, KR152298, KJ923800, KR152300, KJ923770, KJ923773, KJ923780, KR259236, KR186170, KR186180, KR186181, KR186183, KR259243, KR259245 |
| Pseudomonas aeruginosa | Pathogenic; causes infection in immunocompromised individuals; causes endocarditis, osteomyelitis, pneumonia, urinary tract infection, gastrointestinal infections and meningitis | Carrot | SBANHCa1a, SBANHCA14 | Crude, 10-2 | KR152296, KJ923797 |
| Paenibacillus polymyxa | Plant growth promoting bacteria; biocontrol agent and suppresses plant pathogens | Carrot | SBANHCA16 | 10-2 | KJ923799 |
| Paenibacillus illinoisensis | Plant growth promoting rhizobacteria; suppresses the activity of pathogens | Carrot | SBANHCa16a | 10-2 | KR152299 |
| Enterobacter hormaechei | Causes nosocomial infection associated with blood stream infection | Carrot | SBANHCa2a | Crude | KR152301 |
| Staphylococcus sciuri | Animal associated bacteria; causes endocarditis, peritonitis, septic shock, urinary tract infection, endophthalmitis, pelvic inflammatory disease and wound infection | Carrot, Carrot |
SBANHCa7a, SBANHCa14a |
Crude, 10-2 |
KR152302 |
| Bacillus aerophilus | Bioremediation of imidacloprid, a synthetic insecticide | Carrot | SBANSCa3 | Crude | KR186185 |
| Bacillus cereus | Causes food poisoning; vomiting and diarrhoea | Carrot | SBANSCa4 | Crude | KR186186 |
| Microbacterium oleivorans | Plant beneficial bacteria; biocontrol agent to reduce mycotoxin in peanuts, grapes and cereals | Carrot | SBANSCa5 | Crude | KR186187 |
| Arthrobacter nicotianae | Environmentally beneficial bacteria; biodegradation of agro -chemicals | Carrot | SBANSCa6 | Crude | KR186188 |
| Bacillus subtilis | Plant beneficial bacteria; suppresses cucumber Fusarium wilt disease | Carrot | SBANSCa7 | Crude | KR186189 |
| Bacillus flexus | Plant endophytic bacteria; present in roots of wheat crop. | Carrot, Onion, |
SBANSCa10, SBANSO82, |
10-2, Crude, |
KR186190, KR259237, |
| Stenotrophomonas rhizophila | Promotes plant growth; protects against biotic and abiotic stress; associated with human as a nosocomial pathogen | Cucumber, Cucumber, Cucumber, Cucumber, Cucumber, Cucumber, Cucumber, Cucumber |
SBANHCu14a, SBANHCu15, SBANHCu15a, SBANHCu16, SBANHCu17, SBANHCu17a, SBANHCu23, SBANHCu23a |
10-2, 10-2, 10-3 10-3, 10-4, 10-4 10-4, 10-4 |
KR259223, KR259224, KT020946, KR152303, KT020947, KR259225, KT020952, KR152304 |
| Arthrobacter mysorens | Causes erythema with localised skin infection | Cucumber | SBANHCu24 | 10-4 | KR152305 |
| Pseudomonas xanthomarina | Arsenite oxidizing bacteria; helps in biodegradation of petroleum oil | Cucumber, Cucumber, Cucumber, Cucumber, Cucumber |
SBANHCu19, SBANHCu22, SBANHCu25a, SBANSCu19, SBANSCu22 |
10-3, 10-4, 10-4, 10-2, 10-4 |
KT020948, KT020951, KR152306, KR259226, KR259229 |
| Cellulosimicrobium cellulans | Opportunistic pathogen; causes catheter related bacteraemia with short bowel syndrome in children, peritonitis, endocarditis and joint, ocular and soft-tissue infections | Cucumber | SBANHCu10 | Crude | KT020943 |
| Microbacterium schleiferi | Environmentally beneficial bacteria; helps in bioremediation of 1, 3, 5 – TMB (trimethylebenzene) | Cucumber | SBANHCu12 | 10-2 | KT020944 |
| Xanthomonas axonopodis | Plant pathogenic bacteria; causes bacterial pustule disease in soybean | Cucumber | SBANHCu14 | 10-2 | KT020945 |
| Pseudomonas stutzeri | Opportunistic pathogen; causes community acquired pneumonia, meningitis, neonatal septicaemia and knee arthritis in children | Cucumber, Onion, Onion, Onion, Onion, Onion, Tomato |
SBANHCu20, SBANHO3, SBANHO5, SBANHO8, SBANHO13, SBANHO14, SBANHT4 |
10-3, Crude, Crude, 10-2, 10-3, 10-3, Crude |
KT020949, KJ923772, KJ923774, KJ923777, KJ923781, KJ923782, KR186171 |
| Achromobacter xylosoxidans | Opportunistic pathogen; causes malignancies, cardiac disease, meningitis, urinary tract infections, abscesses, osteomyelitis, corneal ulcers, prosthetic valve endocarditis, peritonitis and pneumonia | Cucumber, Cucumber |
SBANHCu21, SBANSCu21 |
10-3, 10-4 |
KT020950, KR259228 |
| Arthrobacter protophormiae | Opportunistic pathogen; present in human skin as commensals; widely distributed in pesticide contaminated agricultural fields | Cucumber | SBANHCu24a | 10-4 | KT020953 |
| Pseudomonas indoloxydans | Useful in biological synthesis of indigo, an important dye-stuff used in textile industries | Cucumber | SBANHCu25 | 10-4 | KT020954 |
| Aeromonas hydrophila | Opportunistic pathogen; causes enteritis, wound infection, septicaemia, pneumonia and conjunctivitis | Cucumber | SBANHCu11 | Crude | KU564062 |
| Geobacillus stearothermophilus | Persists as contaminant in canned food; used as biological indicator for deactivation of pathogens | Cucumber | SBANSCu4 | Crude | KR259230 |
| Staphylococcus haemolyticus | Highly pathogenic; most frequently isolated from blood culture | Cucumber | SBANSCu7 | Crude | KR259231 |
| Bacillus aerius | Halophilic endophytic bacteria; plant growth promotion, phosphate solubilisation, bioremediation of salt affected soil | Cucumber | SBANSCu8 | Crude | KR259232 |
| Bacillus megaterium | Plant beneficial bacteria; helps in nitrogen fixation and promotes plant growth | Cucumber, Cucumber |
SBANSCu9, SBANSCu11 | Crude, 10-2 |
KR259220, KR259233, |
| Microbacterium arborescens | Rhizosphere bacteria of sand dune plant | Cucumber, Cucumber, Onion, Tomato |
SBANSCu10, SBANHCu12a, SBANSO80, SBANHT19 |
Crude, 10-2, Crude, 10-4 |
KR259221, KR259222, KR259235, KR186182 |
| Pseudomonas fluorescens | Plant beneficial rhizobacteria; causes red skin disease with skin hemorrhage and ulceration | Cucumber | SBANSCu20 | 10-3 | KR259227 |
| Xanthomonas fuscans | Plant pathogenic bacteria; infects rice, banana, citrus, bean, tomato, pepper, sugarcane and wheat | Onion, Tomato |
SBANHO6, SBANHT7 |
Crude, Crude |
KJ923775, KR186172 |
| Listeria monocytogenes | Food borne pathogen; causes bacterial meningitis, multiple cerebral ring enhancing lesions | Onion, Tomato, Tomato, Tomato |
SBANHO9, SBANHT9 SBANHT14, SBANHT15 |
10-2, Crude, 10-2, 10-2 |
KJ923778, KR186174, KR186178, KR186179 |
| Bacillus anthracis | Obligate pathogen; causes anthrax: inhalation, gastrointestinal and cutaneous | Onion, Tomato, Tomato |
SBANHO11, SBANHT10, SBANHT12 |
10-2, Crude, 10-2 |
KJ923779, KR186175, KR186176 |
| Staphylococcus epidermidis | Opportunistic pathogen; causes cardiovascular, CNS shunts, joints, blood stream infection and nosocomial infection | Onion, | SBANHO15, | 10-3, | KJ923783, |
| Exiguobacterium acetylicum | Plant beneficial bacteria; in human it causes catheter related bacteraemia | Tomato | SBANHT13 | 10-2 | KR186177 |
| Staphylococcus gallinarum | Rare human pathogen; causes abdominal pain, nausea and weakness | Onion, Tomato |
SBANSO90, SBANHT11 |
10-3, 10-2 |
KR259239, KU564061 |
| Enterococcus faecium | Opportunistic pathogen; causes urinary tract, wound and soft tissue infections, bacteraemia, endocarditis | Onion, Tomato |
SBANSO54, SBANST3 |
Crude, Crude |
KR259234, KR259241 |
| Bacillus aryabhattai | Plant growth promoting bacteria | Onion, Tomato, Tomato |
SBANSO83, SBANST7, SBANST11 |
10-2, Crude, 10-2 |
KR259238, KR259240, KR259244 |
a References of description are given in discussion section
The diversity indices of the endophytic bacterial isolates from the four salad vegetables were calculated using the PAST program (Table 3). The diversity indices were calculated to obtain the diversity of bacterial isolates in the salad vegetables which revealed a lower diversity in tomato, whereas carrot had the highest bacterial diversity compared to other salad vegetables. Moreover, the Dominance (D) and Berger—Parker indices clearly showed that the single taxa of the salad vegetables (tomato) were abundant in the community and the number of isolates are shown in Table 3.
Table 3.
Diversity indices of endophytic bacterial population of the salad vegetables
| Diversity indices/parameters | Formula | Carrot | Cucumber | Onion | Tomato |
|---|---|---|---|---|---|
| Taxa (S) | - | 5 | 4 | 4 | 3 |
| Individuals (n) | - | 31 | 30 | 20 | 21 |
| Dominance (D) | D = Sum (ni/n)2 | 0.3403 | 0.3933 | 0.43 | 0.61 |
| Simpson (1-D) | 1 – D = 1 - Sum (ni/n)2 | 0.6597 | 0.6067 | 0.57 | 0.39 |
| Shannon (H) | H = Sum ((ni/n) In (ni/n)) | 1.245 | 1.123 | 0.9958 | 0.7091 |
| Evenness (E) | E = eH/S | 0.6949 | 0.7684 | 0.6767 | 0.6774 |
| Brillouin (HB) | HB = In (n) – Sum In (ni) | 1.067 | 0.9698 | 0.8154 | 0.5821 |
| Menhinick (db) | db = S/ | 0.898 | 0.7303 | 0.8944 | 0.6547 |
| Margalef (Ma) | Ma = (S-1)/In (n) | 1.165 | 0.882 | 1.001 | 0.6569 |
| Equitability (J) | J = H/Hmax | 0.7738 | 0.81 | 0.7183 | 0.6455 |
| Fisher alpha (FA) | S = α*In (1 + n/α) | 1.687 | 1.24 | 1.504 | 0.9578 |
| Berger-Parker (d) | d = n/nT | 0.4194 | 0.5667 | 0.55 | 0.7619 |
| Chao-1 | Chao1 = S + F1(F1 - 1)/(2 (F2 + 1)), | 5 | 4 | 5 | 3 |
n = number of individuals; n i = number of individuals of taxon i; S = number of taxa; nT = number of individuals in the dominant taxon
Hmax = log S
The isolates were further classified as human pathogens, human opportunistic pathogens, plant pathogens, plant beneficial, plant commensal and environmentally beneficial bacteria and represented as percentage occurrence in each vegetable type (Fig. 4). For this categorization, repeated occurrence of the same species was also counted and represented as percentage to the total population in a given vegetable. It was observed that human opportunistic pathogen was predominant in carrot and onion, whereas plant beneficial bacteria dominated in cucumber and tomato.
Fig. 4.
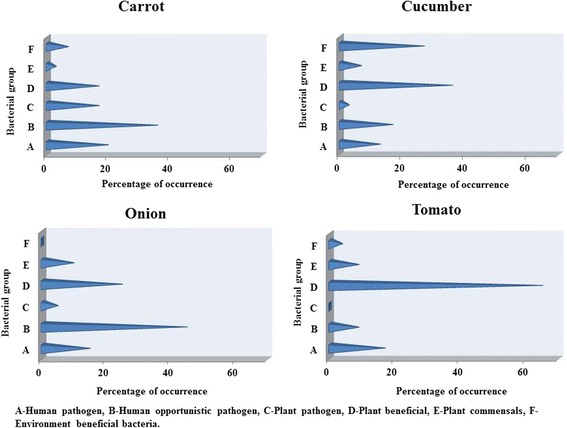
Classification of endophytic bacteria in salad vegetables based on pathogen/non-pathogen nature
Discussion
In spite of potential beneficial aspects of salad vegetables, concerns over their safety and quality have risen since there are number of existing factors responsible for contamination. Fresh salad vegetables have little or no processing steps that can reduce the pathogen levels.
In general, plant harbours diverse bacterial populations both as epiphytes and endophytes, and they are classified based on their characteristics as plant associated bacteria, plant pathogenic, plant growth promoting bacteria and human pathogenic. The presence of bacterial populations as endophytes cannot be removed by washing and thus enters human during consumption of fresh produce as raw salads [9]. Vegetables are considered as the major reservoirs of opportunistic and emerging pathogens due to its diverse microbiome and they are also strongly influenced by biogeographic aspects of farming and food processing practices [10].
In United States, the importance and of fresh produce related infection was realized after the onset of shiga toxin producing E. coli outbreak related to fresh vegetables [11]. The ready-to-eat foods of non-animal origin such as, fruits, vegetables, salads, seeds, nuts, cereals, herbs, spices, fruits and algae had been reported to cause several foodborne outbreaks between 2007 to 2011, in which the major outbreak was with the combination of Salmonella spp. with leafy greens, bulb and stem of vegetables tomatoes and melons, and E. coli with fresh pods, legumes and grains [12]. Consumption of fresh produce raised the occurrence of gastroenteritis, caused by E. coli 0157:H7 and non-typhoidal Salmonella which were traditionally considered as non-hosts for human enteric pathogens. The occurrence of produce-associated outbreaks highlighted our deficiencies in understanding the ecology of enteric pathogens outside human and animal host [13].
The endophytic bacterial isolates observed in our study in tomato, onion, cucumber and carrot belonged to actinobacteria, firmicutes, betaproteobacteria, gammaproteobacteria, bacilli and cocci. The bacteria were categorized based on the known characteristics such as pathogens, non-pathogens and beneficial. Previous reports on the occurrence of human enteric pathogens in salad vegetables were based mostly on enrichment cultures. The bacterial isolates we obtained in the present study were either from crude extract or dilutions up to 10-4. Without enrichment, the average percentage of human pathogens obtained in salad vegetables was 16.25% and of human opportunistic pathogens were 26.5% of the total endophytic isolates. This represents high level of contamination with pathogenic bacteria in the salad vegetables studied. In India, particularly in Tamil Nadu, domestic sewage, industrial and municipal waste water is used for irrigating vegetable crops. This could be one of the reasons for the human pathogenic bacterial load observed in salad vegetables, in addition to possible other reasons including contamination through human and farm animal waste in agricultural lands, post-harvest handling, transport, storage and poor hygiene conditions prevailing in market places.
Viswanathan and Kaur [14] has reported existence of P. aeruginosa, E. coli, S. aureus, Enterobacter sp., Salmonella sp. in carrot. E. coli and S. enterica are known to adapt and persist in plant environment and increase the chance of transmitting to humans via consumption of plants or plant-derived products [15]. The ready-to-eat salads and ready-to-eat sprouts are known to be major vehicle for non-tuberculosis Mycobacteria transmission in humans [16]. Endophytic microorganisms associated with carrot, cucumber, cabbage and onions were analysed by Tayo et al. [1] and E. coli was found to be more predominant, followed by Enterobacter sp., S. aureus, Erwinia sp., Pseudomonas sp. and Salmonella sp.
Among the bacteria that were identified in our study across four vegetables, major genera were Bacillus followed by Staphylococcus, Pseudomonas and Microbacterium. Occurrence of Bacillus aerius was unique to cucumber, similarly B. tequilensis to tomato, B. anthracis and B. aryabhattai to onion. B. pumilus and B. flexus were found to occur in carrot, onion and tomato. We have previously reported the endophytic colonization of B. pumilus in apples, B. flexus and B. subtilis in oranges [17]. B. aryabhattai [18], Terribacillus saccharophilus [19] and B. tequilensis [20] are bacterial strains isolated from the upper atmospheric air of field soils in Japan and 2000-year old Mexican shaft-tomb. B. cereus is an opportunistic pathogen causing food poison [21]. B. anthracis is an obligate pathogen, which survive in several environmental conditions as saprobionts and known to be involved in horizontal gene transfer in plant rhizosphere [22]. Geobacillus stearothermophilus produces lactic acid by fermenting potato starch and potato residues [23]. Paenibacillus illinoisensis has been reported to suppress the activity of antioxidative enzymes in pepper roots caused by Phytophthora capsici infection [24].
In the present study, Staphylococcus sciuri was found to occur only in carrot, S. epidermidis in onion and S. haemolyticus in cucumber. S. aureus was obtained from carrot as well as onion and S. gallinarium from onion as well as tomato. S. aureus is considered as a commensal and a pathogen in human [25]. S. sciuri, animal associated bacteria, is known to be present widely on the skin and mucous surface of pet and farm animals and also in soil, sand, water and marsh grass. The presence of S. sciuri, its adaptation and continuous existence in hospital environment has also been reported [26]. S. epidermidis has been reported as an important human opportunistic pathogen causing infection in cardiovascular, CNS shunts, joints, blood stream etc. [27]. S. haemolyticus is an important hospital acquired pathogen, frequently isolated from human blood cultures [28]. S. gallinarum, a rare human pathogen has been isolated from chicken and saliva of healthy humans [29].
Four among the five species of Pseudomonas observed in our study (P. aeruginosa, P. xanthomarina, P. indoloxydans and P. fluorescens) were from cucumber only. P. stutzeri was found in cucumber and onion. Pseudomonas fluorescens is known to display plant growth promoting rhizobacterial (PGPR) activity [30]. It has been isolated from rhizosphere soils of many crop plants and has received the most research attention among the known PGPR genera. In human, it is a less virulent bacterium causing nosocomial infections in immune-compromised patients [31]. Hu et al. [32] has reported it as a common aquaculture pathogen. P. stutzeri causes knee arthritis in children [33]. P. xanthomarine has been reported as a novel bacteria isolated from marine ascidian [34] and from arsenic contaminated soil [35]. P. stutzeri and P. xanthomarine has the highest biodegrading ability to degrade crude petroleum oil [36]. P. indoloxydans is a lindane (chlorinated pesticide) degrading bacteria isolated from soils of lindane manufacturing industrial site [37].
We also observed Microbacterium oleivorans in carrot, M. schleiferi in cucumber, M. testaceum in tomato and M. arborescens in cucumber and onion. M. testaceum [38] is an environment friendly bacterium which helps in improving the plant growth and yield of crops by suppressing the growth of plant pathogens. M. oleovorans has been reported as a biocontrol agent against Fusarium verticillioides and Aspergillus flavus [39].
Cellulosimicrobium cellulans which was observed in cucumber and tomato tissues is a rare human pathogen, causing septic arthritis mainly in immune-compromised patients [40]. In cucumber plants, it supresses the self-toxic effect by degrading cinnamic acid, benzoic acid, paraaminobenzoic acid and phenol, helps in nutrient absorption rate, adjusts microbial groups in non-rhizospheric soil, thus increasing the number of beneficial bacteria and decreasing fungal growth [41]. Thus, in spite of being a human pathogen, C. cellulans can also act as biocontrol agent for plant pathogens. Stenotrophomonas maltophila has been reported from cucumber rhizosphere involved in supressing Phytophthora crown rot [42]. We observed S. maltophila in carrot and S. rhizophila in cucumber and tomato indicating the species specificity of the bacteria according to the vegetable specific endosphere.
Salmonella enterica is an important foodborne human pathogen, which has the ability to colonize number of plant species such as, Arabidopsis, alfalfa, tomato and various leafy green vegetables [43]. Salmonella triggers mutation in pathogen associated molecular patterns (PAMP) motifs to escape plant defence and it also expresses effectors in the plant tissues to manipulate the plant immune system by triggering PTI (PAMP- triggered immunity) to evolve strategies to avoid or subvert plant immunity [43]. An increase in the growth of S. enteritidis has been reported in melon, watermelon and papaya at different temperatures (10, 20 and 30 °C) [44]. The proliferation of Salmonella is high on red ripped tomato tissues compared to green tomato tissues [45]. It attaches to stem and flowers of tomato plant and remains viable during development of fruit and serves as a route cause for the contamination of tomato fruits [46].
Listeria monocytogenes, Aeromonas hydrophila and Clostridium botulinum have been considered as the most notable pathogens which maintain their infection potential under mild preservation conditions. A. hydrophila has the potential to grow at 0 °C and the temperature of 4–5 °C supports the growth in foods. L. monocytogenes survives in ready-to-eat fresh salads and causes public health risk [47]. Exiguobacterium acetylicum has been reported as an aetiological agent of bacteraemia in humans [48]. Whereas in wheat plants, it improves the growth of seedlings and inhibits the growth of plant pathogens and it is also a cold tolerant bacteria [49]. Bordetella bronchiseptica infects mainly immune-suppressed populations of humans and causes respiratory tract related disease in dogs, cats and rabbits [50]. Arthrobacter protophormiae, when pre-exposed to lower concentration of ONB (O- nitrobenzoate) and PHB (p- hydroxybenzoate) will change its cellular fatty acid profile and adapt itself to survive in high concentration of ONB and PHB [51]. A. mysorens causes erythema characterized by localized skin infection [52]. A. xylosoxidans was reported to be associated with number of outbreaks related to various infections such as malignancies, cardiac disease, meningitis, endocarditis, and pneumonia with number of death cases [53]. Enterobacter hormaechei a unique species was first identified in 1989 followed by several outbreaks on sepsis in neonatal intensive care units in Brazil and USA [54]. E. aerogenes and E. cloacae has been reported in hospital acquired infections in Europe and France, and they also have strong antibiotic resistance, which helps in colonizing multiple environments and hosts [55]. E. asburiae, epiphytic bacteria, exists as parasitic or commensal with in plant host and produce virulence factors based on their quorum sensing activity and has been isolated from soil, water, food products and lettuce leaves [56]. The type III secretion system (T3SS) encoded by hrp gene in plant associated bacteria Xanthomonas fuscans sub sp. fuscans is responsible for colonizing bean plants and causing bacterial blight disease [57]. Plant pathogenic bacterium Xanthomonas axonopodis cause bacterial pustule disease in soybean [58].
Surette et al. [59] enumerated the endophytic bacteria in carrot and found 83% of the strains were plant growth promoting bacteria. In our study, carrot contained more of human opportunistic pathogens followed by human pathogens, plant beneficial and plant pathogenic bacteria. In carrot, population of human opportunistic pathogens were high and approximately double the number of plant pathogens and plant beneficial bacteria. The scenario was the other way around in cucumber, where plant beneficial bacterial population was double and more than double the population of human opportunistic and human pathogens, respectively. In onion, human opportunistic pathogen population was high compared to plant beneficial. Tomato had a comparatively very high population of beneficial bacteria in which the human pathogens and plant pathogens were found to be drastically reduced. Comparing the functional diversity in all four vegetables, existence of high population of plant beneficial bacteria has suppressed the growth of human pathogens, human opportunistic pathogens and plant pathogens. Whether the difference in bacterial population between pathogens and non-pathogens is due to the suppression effect of plant beneficial bacteria on human opportunistic pathogens or suppression effect of human opportunistic pathogens on plant beneficial bacteria needs further in-depth analysis of their interaction in this ecological niche.
It is also interesting to note that population of human pathogens remain more or less similar (approx. 20% to the total) in carrot and tomato, irrespective of reasonably high occurrence of human opportunistic pathogens (carrot) and plant beneficial bacteria (tomato). However, the occurrence of human pathogens was below 20% when the combined population of human opportunistic pathogens and plant beneficial bacteria (onion) or plant beneficial and environmentally beneficial bacteria (cucumber) was above 50%. Percentage of human pathogens was less than 20%, only when population of plant beneficial bacteria was above 20%. Population of human opportunistic pathogens was 45, 36, 17 and 8% respectively in onion, carrot, cucumber and tomato compared to the population percentage of 25, 17, 36 and 63 of plant beneficial bacteria in these vegetables. There is a clear evidence for the suppressive effect of plant beneficial bacteria on human opportunistic pathogens in cucumber and tomato. When the population of plant beneficial bacteria was above 17% (onion – 25; cucumber – 36; tomato – 63), the plant pathogens were accordingly suppressed (onion – 5; cucumber – 3; tomato – 0). Although pathogen population was less (5%) due to higher number of human opportunistic pathogens (45%), as observed in onion, similar trend was not observed in cucumber and tomato where the suppression of plant pathogens (3 and 0% respectively) was parallel to human opportunistic pathogens (17 and 8% respectively). This indicates the suppressed population of these two groups of bacteria are independent of each other and dependent on plant beneficial bacteria. It can be concluded that plant beneficial bacteria suppresses both plant and human opportunistic pathogens. Occurrence of commensal bacteria lacks correlation with population of other categories of bacteria. However, commensal bacteria were found to be suppressed when all other bacteria contribute reasonably to the total population as observed in carrot. Higher occurrence of human opportunistic bacteria (45% in onion) was found to have a significant effect on environmentally beneficial bacteria. Previous reports indicate the difference in composition of plant microbiome based on the soil contamination with pollutants as well as organic cultivation and pesticide usage [60, 61]. Hence, the observed composition of the vegetable bacteriome would be due to the prevailing soil conditions such as pollutant and pesticide level. Further studies on the bacteriome of these vegetables from different agro-climatic zones, soils and cultivation conditions would be useful in understanding the basal level bacterial species specific to these vegetables. In addition, such studies will also help to identify soil, climatic zone and pollutant specific species which have gained endophytic status.
Conclusions
The complex microbial community analysis and their interactions with plant have greater potential to elucidate interactions between host plants and bacteria as well as bacteria—bacteria interactions. Elucidating the cross-talk between different bacterial communities would not only help in understanding the interaction but also to evolve new biocontrol agents for plant and human pathogens. Based on the observations on relative population density of different categories of the bacteriome in salad vegetables, we propose that there is a greater potential to study the native endophytic plant beneficial bacteria and to develop them as biocontrol agents against not only the plant pathogens, but also the human pathogens that are harboured by plants.
Acknowledgement
We acknowledge the management of VIT University, Vellore, India for providing the facilities, laboratory space and support in carrying out the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and material
The datasets generated and/or analysed during the current study are available in the GenBank repository, https://www.ncbi.nlm.nih.gov/nuccore/?term=nithya+and+Babu+VIT+University.
Authors’ contributions
AN: Performed the sampling, microbiological work, amplification and also contributed in manuscript writing. SB: Conceived the concept, mentored the work, contributed in sequence analysis and submission to GenBank and contributed in manuscript writing. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AIA
Aeromonas Isolation Agar
- BLAST
Basic Local Alignment Search Tool
- C
Citrate
- CA
Certemaid Agar
- CDC
Center for Disease Control and Prevention
- CFU
Colony Forming Units
- CNS
Central Nervous System
- CSPI
Center for Science in the Public Interest
- EMBA
Eosin Methylene Blue Agar
- FDA
US Food and Drug Administration
- I
Indole
- M
Motility
- MR
Methyl Red
- MSA
Mannitol Salt Agar
- N
Nitrate
- O
Oxidase
- ONB
O- nitrobenzoate
- PAMP
Pathogen Associated Molecular Patterns
- PHB
P- hydroxybenzoate
- PTI
PAMP- triggered immunity
- SSA
Salmonella—Shigella Agar
- T3SS
Type III Secretion System
- TAL
Transcription Activator Like effector
- VP
Voges—Proskauer
- WHO
World Health Organization
Additional file
(a). Morphological and biochemical characterization of bacterial isolates from carrot. (b). Morphological and biochemical characterization of bacterial isolates from cucumber. (c). Morphological and biochemical characterization of bacterial isolates from onion. (d). Morphological and biochemical characterization of bacterial isolates from tomato. (DOC 233 kb)
References
- 1.Tayo BC, Odu NN, Esen CU, Okonko IO. Microorganisms associated with spoilage of stored vegetables in Uyo Metropolis, Akwa Ibom state, Nigeria. Nat Sci. 2012;10:23–32. [Google Scholar]
- 2.Vaz BM, Fink RC, Gonzalez FD, Sadowsky MJ. Minireview enteric pathogen-plant interactions: molecular connections leading to colonization and growth and implications for food safety. Microbes Environ. 2014;29:123–135. doi: 10.1264/jsme2.ME13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leff JW, Fierer N. Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0059310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rovira J, Cencic A, Santos E, Jakobsen M. Biological hazards. In: Luning PA, Devlieghere F, Verhe R, editors. Safet in the agri–food chain. The Netherlands: Wageningen Academic Publishers; 2006. [Google Scholar]
- 5.Wiedemann A, Payant IV, Chausse AM, Schikora A, Velge P. Interactions of Salmonella with animals and plants. Front Microbiol. 2015;5:1–18. doi: 10.3389/fmicb.2014.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 7.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammer O, Harper DAT, Rayn PD. PAST: Paleontological statistics softer pakage for education and data analysis. Palaeontol Electronic. 2001;4:1–9. [Google Scholar]
- 9.Jackson CR, Randolph KC, Osborn LO, Tyler HL. Culture dependent and independent analysis of bacterial communities associated with commercial salad leaf vegetables. BMC Microbiol. 2013;13:1–12. doi: 10.1186/1471-2180-13-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg G, Erlacher A, Smalla K, Krause R. Vegetables microbiomes is there a connection among opportunistic infections, human health and our gut feeling? Microb Biotechnol. 2014;7:487–495. doi: 10.1111/1751-7915.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivas M, Chines I, Miliwebsky E, Masana M. Risk factors for Shiga toxin –producing Escherichia coli associated human diseases. Microbiol Spectrum. 2014;2:1–14. doi: 10.1128/microbiolspec.EHEC-0002-2013. [DOI] [PubMed] [Google Scholar]
- 12.Felicio MIDS, Hald T, Liebana E, Allende A, Hugas M, The CN. Johannersen GK, Niskanen T, Uyttendaele M, Mchauchlin J. Risk ranking pathogens in ready-to-eat unprocessed foods of non – animal origin (FONAO) in the EV: Initial evaluation using outbreak data (2007-2011) Int J Food Microbiol. 2015;195:9–19. doi: 10.1016/j.ijfoodmicro.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Teplitski M, Barak JD, Schneider KR. Human enteric pathogens in produce :un-answered ecological questions with direct implications for food safety. Curr Opin Biotechnol. 2009;20:166–171. doi: 10.1016/j.copbio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan P, Kaur R. Prevalence and growth of pathogens on salad vegetables, fruits and sprouts. Int J Hyg Environ Health. 2001;203:205–213. doi: 10.1078/S1438-4639(04)70030-9. [DOI] [PubMed] [Google Scholar]
- 15.Overbeek LSV, Doom JV, Wichers JH, Amerongen AV, Roermund HJWV, Willemsen PTJ. The arable ecosystem as bottle ground for emergence of new human pathogens. Front Microbiol. 2014;5:1–17. doi: 10.3389/fmicb.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortes JFC, Monter NL, Cueto ALC, Rangel LPS, Reptto ACH, Hernandez DL, Gutierrez SR, Rendon EF, Merchand JAG. Microbiological Quality of ready-to-eat vegetables collected in Mexico city: Occurrence of aerobic-mesophilic bacteria, fecal coliforms and potentially pathogenic nontuberculous Mycobacteria. BioMed Res Int. 2015;2015:789508. [DOI] [PMC free article] [PubMed]
- 17.Phukon M, Sahu P, Srinath R, Nithya A, Babu S. Unusual occurance of Staphylococcus warneri as endophyte in fresh fruits along with usual Bacillus spp. J Food Safety. 2013;33:102–106. doi: 10.1111/jfs.12028. [DOI] [Google Scholar]
- 18.Shivaji S, Chaturvedi P, Begum Z, Pindi PK, Manorama R, Padmanaban A, Shouche YS, Pawar S, Vaishampayan P, Dutt CBS, Datta GN, Manchanda RK, Rao UR, Bhargava PM, Narlikar JV. Janibacter hoylei sp. nov., Bacillus isronensis sp. nov. and Bacillus aryabhattai sp. nov., isolated from cryotubes used for collecting air from the upper atmosphere. Int J Syst Evol Microbiol. 2009;59:2977–2986. doi: 10.1099/ijs.0.002527-0. [DOI] [PubMed] [Google Scholar]
- 19.An SY, Asahara M, Goto K, Kasai H, Yokota A. Terribacillus saccharophilus gen. nov., sp. Nov. and Terribacillus halophilus sp. nov., spore-forming bacteria isolated from field soil in Japan. Int J Syst Evol Microbiol. 2007;57:51–55. doi: 10.1099/ijs.0.64340-0. [DOI] [PubMed] [Google Scholar]
- 20.Gatson JW, Benz BF, Chandrasekaran C, Satomi M, Venkateswaran K, Hart ME. Bacillus tequilensis sp. nov., isolated from a 2000-year-old Mexican shaft-tomb, is closely related to Bacillus subtilis. Int J Syst Evol Microbiol. 2006;56:1475–1484. doi: 10.1099/ijs.0.63946-0. [DOI] [PubMed] [Google Scholar]
- 21.Raymond B, Wyres KL, Sheppard SK, Ellis RJ, Bonsall MB. Environmental factors determining the epidemiology and population genetic structure of Bacillus cereus group in the field. PLoS Pathog. 2010;6:1–13. doi: 10.1371/journal.ppat.1000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganz HH, Turner WC, Brodie EL, Kusters M, Shi Y, Sibanda H, Torok T, Getz WM. Interactions between Bacillus anthracis and plants may promote anthrax transmission. PLoS Negl Trop Dis. 2014;8:2903. doi: 10.1371/journal.pntd.0002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smerilli M, Neureiter M, Wurz S, Haas C, Fruhauf S, Fuchs W. Direct fermentation of potato starch and potato residues to lactic acid by Geobacillus stearothermophilus under non-sterile conditions. J Chem Technol Biotechnol. 2015;90:648–657. doi: 10.1002/jctb.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung WJ, Jin YL, Park RD, Kim KY, Lim KT, Kim TM. Treatment of Paenibacillus illinoisensis suppresses the activities of antioxidative enzyme in pepper roots caused by Phytophthora capsici infection. World J Microbiol Biotechnol. 2006;22:901–907. doi: 10.1007/s11274-006-9131-7. [DOI] [Google Scholar]
- 25.Chaves-Moreno D, Wos-Oxley ML, Jauregui R, Medina E, Oxley APA, Pieper DH. Exploring the transcriptome of Staphylococcus aureus in its natural niche. Sci Rep. 2016; 6. doi: 10.1038/srep33174. [DOI] [PMC free article] [PubMed]
- 26.Dakic I, Morrison D, Vukovic D, Savic B, Shittu A, Jezek P, Hauschild, Stepanovic S. Isolation and molecular characterization of Staphylococcus sciuri in the hospital environment. J Clin Microbiol. 2005;43:2782–2785. doi: 10.1128/JCM.43.6.2782-2785.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buttner H, Mack D, Rohde H. Structural basis of Staphylococcus epidermidis biofilm formation: mechanisms and molecular interactions. Front Cell Infect Microbiol. 2015;5:1–15. doi: 10.3389/fcimb.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barros EM, Ceotto H, Bastos MCF, Santos KRN, Giambiagi M. Staphylococcus haemolyticus as an important hospital pathogen and carrier of methicillin resistance genes. J Clin Microbiol. 2011;50:166–168. doi: 10.1128/JCM.05563-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tibra NK, Jalali S, Reddy AK, Narayanan R, Agarwal R. Traumatic endophthalmitis caused by Staphylococcus gallinarum. J Med Microbiol. 2010;59:365–366. doi: 10.1099/jmm.0.011551-0. [DOI] [PubMed] [Google Scholar]
- 30.Santoro MV, Bogino PC, Nocelli N, Cappellari LR, Giordano WF, Banchio E. Analysis of plant growth-promoting effects of fluorescent Pseudomonas strains isolated from Mentha piperita rhizosphere and effects of their volatile organic compounds on essential oil composition. Front Microbiol. 2016. http://dx.doi.org/10.3389/fmicb.2016.01085. [DOI] [PMC free article] [PubMed]
- 31.Wong V, Levi K, Baddal B, Turton J, Boswell TC. Spread of Pseudomonas fluorescens due to contaminated drinking water in a bone marrow transplant unit. J Clin Microbiol. 2011;49:2093–2096. doi: 10.1128/JCM.02559-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu YH, Dang W, Sun L. The ton B- dependent outer membrane receptor of Pseudomonas fluorescens: virulence and vaccine potential. Arch Microbiol. 2012;194:795–802. doi: 10.1007/s00203-012-0812-3. [DOI] [PubMed] [Google Scholar]
- 33.Miron D, Keness Y, Bor N, Spiegel R, Horowitz Y. Pseudomonads stutzeri knee arthritis in a child: case report and review. J Pediatr Orthop B. 2007;16:419–421. doi: 10.1097/BPB.0b013e3282f058bb. [DOI] [PubMed] [Google Scholar]
- 34.Romanenko LA, Uchino M, Falsen E, Lysenko AM, Zhukova NV, Mikhailov VV. Pseudomonas xanthomarine sp. a novel bacterium isolated from marine ascidian. J Gen Appl Microbiol. 2005;51:65–71. doi: 10.2323/jgam.51.65. [DOI] [PubMed] [Google Scholar]
- 35.Koechler S, Ploetze FA, Armanet CB, Chollet FG, Salmeron AH, Jost B, Lievremont D, Philipps M, Plewniak F, Bertin PN, Lett MC. Constituvative arsenite oxidase expression detected in arsenic-hypertolerant Pseudomonas xanthomarine S11. Res Microbiol. 2015;166:205–214. doi: 10.1016/j.resmic.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Sheshtawy HSE, Khalil NM, Ahmed W, Abdallah RI. Monitoring of oil pollution at Gemsa Bay and bioremediation capacity of bacterial isolates with bio-surfactants and nanoparticles. Marine Poll Bull. 2014;87:191–200. doi: 10.1016/j.marpolbul.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 37.Manickam N, Ghosh A, Jain RK, Mayilraj S. Description of a novel indole-oxidizing bacteria Pseudomonas indoloxydans sp. nov., isolated from pesticide-contaminated site. Syst Appl Microbiol. 2008;31:101–107. doi: 10.1016/j.syapm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Morohoshi T, Wang WZ, Someya N, Ikeda T. Genome sequence of Microbacterium testaceu StLB037, an N-Acylhomoserine Lactone-Degrading bacterium isolated from potato leaves. J Bacteriol. 2011;193:2072–2073. doi: 10.1128/JB.00180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chulze SN, Palazzini JM, Torres AM, Barros G, Ponsone ML, Geisen R, Schmidt HM, Kohl J. Biological control as a strategy to reduce the impact of mycotoxins in peanut, grapes and cereals in Argentina. Food Addit Contam Part A. 2015;32:471–479. doi: 10.1080/19440049.2014.984245. [DOI] [PubMed] [Google Scholar]
- 40.Checa CM, Chaparro LC, Ruiz JP, Monje AP, Alexander JLR, Salvatierra J, Raya E. Septic arthritis due to Cellulosimicrobium cellulans. J Clin Microbiol. 2011;49:4391–4393. doi: 10.1128/JCM.05058-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu GH, Xiey H, Chen YH, Chen YF, Cheng P. Mitigating the repress of cinnamic acid to cucumber growth by microbial strain. Wei Sheng Wu Xue Bao. 2006;46:934–938. [PubMed] [Google Scholar]
- 42.Islam S, Akanda AK, Prova A, Islam MT, Hossaim MM. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front Microbiol. 2016;6:1–12. doi: 10.3389/fmicb.2015.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia AV, Charrier A, Schilcora A, Bigeard J, Pateyron S, Moreau MLT, Evrard A, Mithofer A, Magniette MLM, Payant IV, Hirt H. Salmonella enteric flagellin is recognized via FLS2 and activities PAMP-triggered immunity in Arabidopsis thaliana. Mol Plant. 2014;7:657–674. doi: 10.1093/mp/sst145. [DOI] [PubMed] [Google Scholar]
- 44.Penteado AL, Leitao MFF. Growth of Salmonella Enteritidis in melon, water melon and papaya pulp stored at different time and temperature. Food Control. 2004;15:363–373. doi: 10.1016/S0956-7135(03)00099-9. [DOI] [Google Scholar]
- 45.Marvasi M, Hochmuth GJ, Giurcanu MC, George AS, Noel JT, Bartz J, Teplitski M. Factors that affect proliferation of Salmonella in tomatoes post-harvest: the roles of seasonal effects, irrigation regime, crop and pathogen genotype. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo X, Chen J, Brackett RE, Beuchat LR. Survival of Salmonellae on and in tomato plants from the time of inoculation at flowering and early stages of fruit development through fruit ripening. Appl Environ Microbiol. 2001;67:4760–4764. doi: 10.1128/AEM.67.10.4760-4764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montero D, Bodero M, Riveros G, Lapierre L, Gaggero A, Vidal RM, Vidal M. Molecular epidemiology and genetic diversity of Listeria monocytogenes isolated from wide variety of ready-to-eat foods and their relationship to clinical strains from listeriosis outbreaks in Chile. Front Microbiol. 2015;6:1–8. doi: 10.3389/fmicb.2015.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keynan Y, Weber G, Sprecher H. Molecular identification of Exiguobacterium acetylicum as the aetiological agent of bacteraemia. J Med Microbiol. 2007;56:563–564. doi: 10.1099/jmm.0.46866-0. [DOI] [PubMed] [Google Scholar]
- 49.Selvakumar G, Joshi P, Nazim S, Mihra PK, Kundu S, Gupta HS. Exigubacterium acetylicum train 1P (MTCC 8707) a novel bacterial antagonist from the North Western Indian Himalayas. World J Microbiol Biotechnol. 2009;25:131–137. doi: 10.1007/s11274-008-9874-4. [DOI] [Google Scholar]
- 50.Yacoub AT, Katayama M, Tran J, Zadikany R, Kandula M, Greene J. Bordetella bronchiseptica in the immunosuppressed population – A case series and review. Mediterr J Hematol Infect Dis. 2014;6:1–7. doi: 10.4084/MJHID.2014.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma NK, Pandey J, Gupta N, Jain RK. Growth and physiological response of Arthrobacter protophormiae RKJ100 toward higher concentrations of o-nitrobenzoate and p-hydroxybenzoate. FEMS Microbiol Lett. 2007;271:65–70. doi: 10.1111/j.1574-6968.2007.00697.x. [DOI] [PubMed] [Google Scholar]
- 52.Imirzalioglu C, Hain T, Hossain H, Chakraborty T, Domann E. Erythema caused by localised skin infection with Arthrobacter mysorens. BMC Infect Dis. 2010;10:1–4. doi: 10.1186/1471-2334-10-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duggan JM, Goldstein SJ, Chenoweth CE, Kauffman CA, Bradley SF. Achromobacter xylosoxidans bacteremia: report of four cases and review of the literature. Clin Infect Dis. 1996;23:569–576. doi: 10.1093/clinids/23.3.569. [DOI] [PubMed] [Google Scholar]
- 54.Townsend SM, Hurrell E, Barron JC, Carrillo CL, Forsythe SJ. Characterization of an extended-spectrum beta-lactamase Enterobacter hormaechei nosocomial outbreak, and other Enterobacter hormaechei misidentified as Cronobacter (Enterobacter) sakazakii. Microbiology. 2008;154:3659–3667. doi: 10.1099/mic.0.2008/021980-0. [DOI] [PubMed] [Google Scholar]
- 55.Davin-Regli A, Pages JM. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol. 2015;6:1–10. doi: 10.3389/fmicb.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau YY, Yin WF, Chan KG. Enterobacter asburiae strain L1: Complete genome and whole genome optical mapping analysis of a quorum sensing bacterium. Sensors. 2014;14:13913–13924. doi: 10.3390/s140813913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Broughton WJ, Hern G, Blair M, Beebe S, Gepts P, Vanderleyden J. Beans (Phaseolus spp.) – model food legumes. Plant Soil. 2003;252:55–128. doi: 10.1023/A:1024146710611. [DOI] [Google Scholar]
- 58.Lee JH, Shin H, Park HJ, Ryu S, Han SW. Draft genome sequence of Xanthomonas axonopodis pv. Glycines 8ra possessing transcription activator – like affectos used genetic engineering. J Biotechnol. 2014;179:15–16. doi: 10.1016/j.jbiotec.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Surette MA, Sturz AV, Lada RR, Nowak J. Bacterial endophytes in processing carrots (Daucus carota L. var. sativus): their localization, population density, biodiversity and their effects on plant growth. Plant Soil. 2003;253:281–390. doi: 10.1023/A:1024835208421. [DOI] [Google Scholar]
- 60.Xia Y, DeBolt S, Dreyer J, Scott D, Williams MA. Characterization of culturable bacterial endophytes and their capacity to promote plant growth from plants grown using organic or conventional practices. Front Plant Sci. 2015;6:490. doi: 10.3389/fpls.2015.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemactud R, Shen SY, Lau M, Fulthorpe R. Bacterial endophytes isolated from plants in natural oil seep soils with chronic hydrocarbon contamination. Front. Microbiol. 2016; https://doi.org/10.3389/fmicb.2016.00755. [DOI] [PMC free article] [PubMed]


